We study how AMPK, the master regulator of metabolism, intersects with and thereby, shapes biological processes beyond typical metabolic diseases. Our focus is particularly on how AMPK’s regulation of energy homeostasis alters the outcomes of immune responses, tumour growth and the aging trajectory.
Our central focus is the metabolic enzyme, AMP-activated protein kinase (AMPK), an ancient energy sensor that monitors and maintains cellular energy levels by inhibiting energy-consuming processes and promoting energy-generating pathways when energy is scarce. An important aspect of this function is AMPK’s role in safeguarding mitochondria, often considered the cell’s powerhouse, essential for production of energy.
While AMPK’s role in metabolic conditions like diabetes and obesity is well studied, its impact on other biological processes, such as immunity and aging, remains less well understood. Since energy is fundamental to all cellular functions, understanding AMPK’s influence on energy intensive processes, where disruptions in energy balance can have a significant impact, is crucial.
Although cancer immunotherapy, such as immune checkpoint blockade, has transformed cancer treatment, most patients do not respond. Evidence suggests that energy dysregulation may influence various aspects of the immune response. Our lab is investigating whether AMPK and its regulation of mitochondrial dynamics can be harnessed to enhance cancer immunotherapy.
Aging and neurodegeneration are also associated to defective mitochondria recycling machinery (a process known as autophagy that relies on lysosomes) and mitochondrial dysfunction. Our lab is examining how AMPK’s role in promoting both lysosome and mitochondra production – demonstrated in our previous findings – could have significant implications for the treatment of ageing related conditions such as Parkinson’s.
The ability to adapt to prolonged nutrient deprivation is essential for the survival of all organisms. AMPK is a highly conserved energy sensor in eukaryotes, rapidly activated when ATP levels decline, such as during nutrient shortage or mitochondrial dysfunction. AMPK activation occurs within minutes of energy stress and through phosphorylation of its substrates, shifts metabolism from an anabolic to a catabolic state, restoring metabolic balance. Additionally, AMPK drives long-term metabolic adaptation by downregulating biosynthetic genes and upregulating genes that promote lysosomal and mitochondrial biogenesis.
Our recent published work (Malik et al., 2023) identified FNIP1 as a key AMPK substrate that controls TFEB translocation to the nucleus, establishing AMPK phosphorylation of FNIP1 as a critical event required for lysosomal and mitochondrial gene expression after energy stress. This discovery also addresses the broader biological question of how mitochondrial damage triggers a coordinated response for repair and replacement of damaged mitochondria.
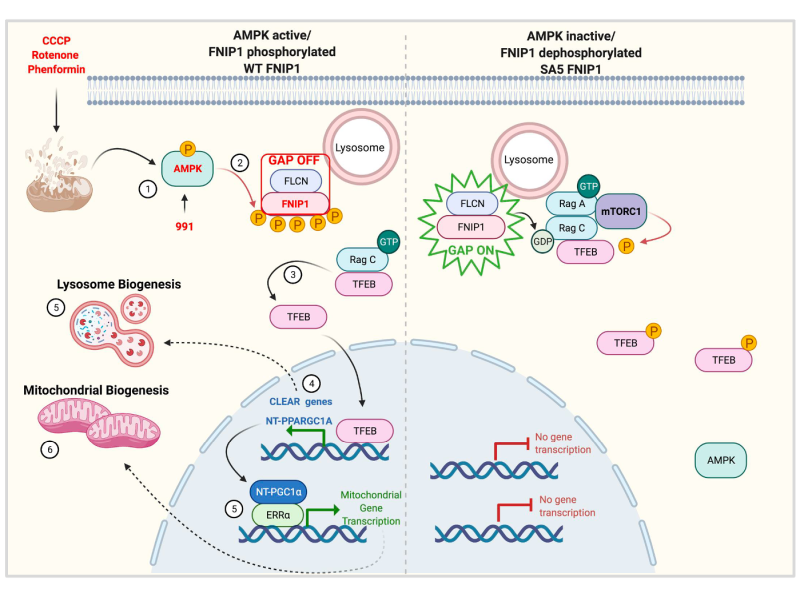
These findings open therapeutic avenues for diseases associated with mitochondrial dysfunction, from neurodegeneration to cancer and set the stage for exploring AMPK’s role in energy-demanding processes such as immune response.
Building on exciting preliminary data and centred on AMPK’s role in mitochondrial homeostasis, we aim to understand how AMPK driven metabolism intersects with innate and adaptive immunity (particularly in the context of cancer) and neurodegenerative processes.
Our key areas of investigation include:
Immunity
Aging
Malik N, Ferriera B, Hollstein P, Curtis S D, Trefts E, Weiser-Novak S, Yu J, Gilson R, Hellbery K, Fang L, Sheridan A, Hah N, Shadel G, Manor U, Shaw R J. Induction of lysosomal and mitochondrial biogenesis by AMPK phosphorylation of FNIP1. Science. 2023 Apr 21; Vol. 380: no. 6642
Hung CM, Lombardo P, Malik N, Brun S N, Hellberg K, Van Nostrand J L, Garcia D, Baumgart J, Diffenderfer K, Asara J M, Shaw R J. AMPK/ ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Science Advances. 2021 Apr 7; Vol. 7, no. 15
Malik N, Nirujogi R, Peltier J, Macartney T, Whightman M, Prescott A R, Gourlay R, Trost M, Alessi D, Karapetsas T. Phosphoproteomics reveals that the hVPS34 regulated SGK3 kinase specifically phosphorylates endosomal proteins including Syntaxin-7, Syntaxin-12, RFIP4 and WDR44. Biochem J. 2019 Oct 30; 475(1):117-135.
Malik N, Macartney T, Hornberger A, Anderson K, Prescott A, Alessi D. Mechanism of Activation of SGK3 by growth factors via the Class I and Class III pathways. Biochem J. 2018 Jan 2;475(1):117-135.
Malik N., Vollmer S., Nanda S.K., Lopez-Pelaez M., Prescott A., Gray N., Cohen P. Suppression of interferon β gene transcription by inhibitors of bromodomain and extra-terminal (BET) family members. Biochem J. 2015 468: 363–372.
Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, Shpiro N, Ward R, Cross D, Ganley IG, Alessi D Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J. 2014 463: 413-427