Scientists uncover the presence of histones in bacteria, reshaping existing understandings of bacterial DNA organization.
By LMS Staff Member
October 9, 2023
Time to read: 3 minutes
New research featured in Nature Microbiology could potentially be rewriting biology textbooks, proving the existence of the DNA packaging protein called histone in bacterial cells. What’s more, they uncovered a new binding mode of this protein.
The study, led by groups at the Medical Research Council Laboratory of Medical Sciences (MRC LMS), based at Imperial College London, with collaborators from the University of Nottingham, the University of Colorado Boulder, and the Institut Pasteur in Paris is the first study that delves into the structure and function of histones in bacteria.
“Histones have long been believed to exist exclusively in archaea and eukaryotes. ”
said Dr Tobias Warnecke, leader of the MRC LMS Molecular Systems Research Group.
“Computational analyses of bacterial genomes in recent years have suggested that there might also be histones in bacteria. We have now shown that this is indeed the case. What’s more, it turns out that these histones are not just bit players, but major factors in how these bacteria organize their DNA.”
Histones are proteins that bind to DNA and, in humans for example, are critical to gene regulation, because they affect which bits of the DNA can be read at a given time Controlling access to DNA is important because, to build different cell types or respond to the environment in the right way, cells need to express only some, not all of their genes.
Bacteria, were thought to rely on other proteins to orchestrate access and organize their DNA. These proteins are less essential as deleting them doesn’t lead to cell death.
However, this recent study uncovered that some bacteria do use histones as their key packaging tools. By scraping through a diverse database of bacterial genomes, researchers revealed hundreds of histone-fold proteins in bacteria.
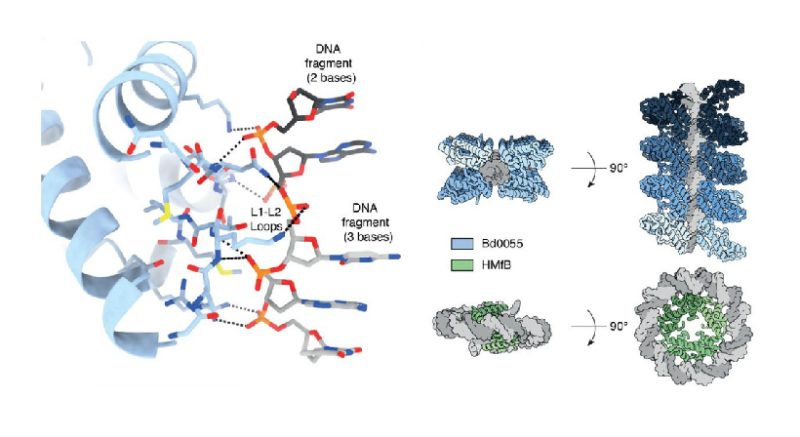
Researchers then studied the proteins in two types of bacteria. One, called Bdellovibrio bacterivorous, attacks other bacteria and has been hailed as a potential alternative to antibiotics. The other, Leptospira interrogans, is a pathogen that infects humans and animals, causing Weil’s disease, a type of a bacterial infection. The researchers found that, in both bacteria, histones are an integral part of their chromatin and essential throughout the cell’s life cycle.
Further study of Bdellovirbrio bacterivorous revealed that the histones bind DNA in a unique fashion. In all other organisms studied so far, including humans, histones form a complex around which DNA wraps. The bacterial histone from Bdellovibrio bacteriovorus, called Bd0055, on the other handbound DNA by attaching to the outside of the DNA strands, forming a shield that completely encased DNA. This is completely different from the conventional way where DNA is wrapped around the histone surface.
“Our findings expand our understanding of histone structure and function across diverse life forms. We eagerly anticipate future research exploring the functions and implications of histones in a wider array of bacterial species.”
said Antoine Hocher, first author of the study and post-doc researcher at LMS.
Read the paper here.