“What is the link between metabolic disease and cancer?”
“We explore complex interactions between metabolic disease and cancer progression”
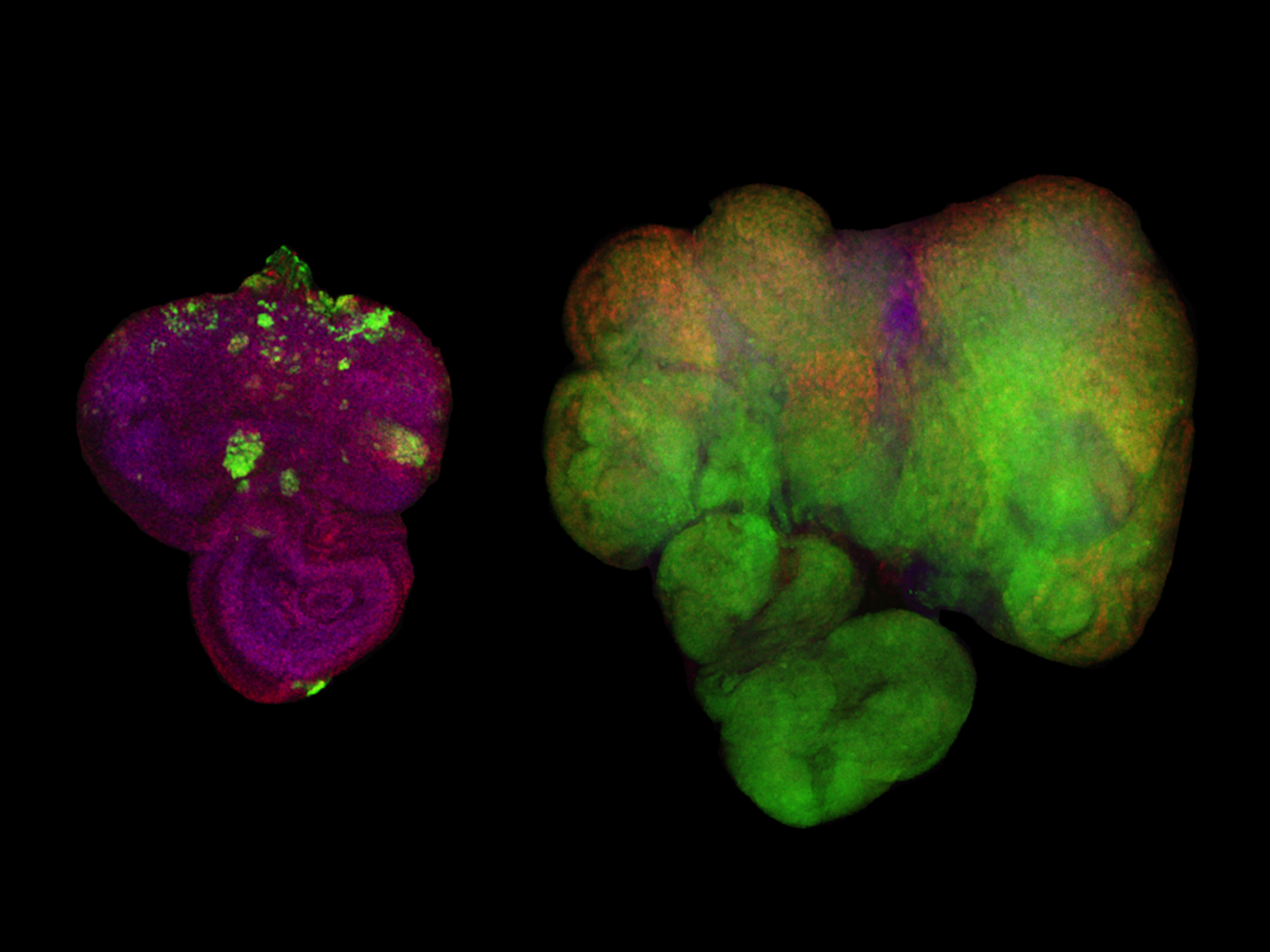
Diet-induced obesity promotes tumor overgrowth in Drosophila. Ras/Src-activated tumors (labeled with GFP; green) in the Drosophila eye epithelial tissues of animals raised on a control diet (left) and a high-sugar diet (right).

Choutka C, Cabrera C, Hirabayashi S. (2022) Embracing complexity in Drosophila cancer models. Dis Model Mech 15 (3). https://doi.org/10.1242/dmm.049513
Newton H, Wang Y, Camplese L, Mokochinski JB, Kramer HB, Brown AEX, Fets L, Hirabayashi S. (2020). Systemic muscle wasting and coordinated tumour response drive tumourigenesis. Nature Communications 11, 4653.
Hirabayashi S and Cagan R. (2020). Systemic Regulation of Local Cell Competition. Developmental Cell 53, 371-372.
Hirabayashi S (2016). The interplay between obesity and cancer: a fly view. Dis Model Mech 9, 917-926.
Hirabayashi S, Cagan RL. (2015). Salt-inducible kinases mediate nutrient-sensing to link dietary sugar and tumorigenesis in Drosophila. eLife 4, e08501.
Hirabayashi S, Baranski TJ, Cagan RL. (2013). Transformed Drosophila Cells Evade Diet-Mediated Insulin Resistance through Wingless Signaling. Cell 154, 664-675.