Redox signalling is a critical process within the cell essential for key signalling pathways. We are working to better understand the molecular processes that guide its role in metabolism, disease, and ageing, and to uncover novel pathways and potential therapeutic targets.
“Can we influence cell signalling to help combat diseases?”
Redox signalling is a critical process within the cell essential for key signalling pathways. It plays a part in gene expression, modulates a number of metabolic pathways and keeps the cell biochemically balanced.
When redox signalling is disrupted, it can cause issues for the cell and the organism as a whole. Dysregulation of redox signalling is linked to many age-related diseases, as well as the aging process itself.
We are working to better understand how redox signalling is controlled, as well as how it can be influenced by drugs and diet. We recently demonstrated that sugar-induced obesity and insulin resistance may not necessarily lead to a shortened lifespan, and could be balanced by an increased intake of water.
We use the fruit fly Drosophila as in vivo systems through which we conduct our research. We use a combination of biochemical and genetic approaches to find out more about the physiological importance of redox signalling.
For studies focussing the links between redox signalling and diet for example, we place Drosophila on different feeding regimes to study the changes in their metabolism.
Ultimately, our goal is to uncover novel pathways and potential therapeutic targets for health and longevity benefits, including combating obesity, insulin resistance, and even aging.
“Our research focuses on investigating the role of redox signalling in metabolism, disease and ageing.”
Redox signalling is an important modulator of diverse biomedically relevant metabolic pathways (Figure 1). Consequently, dysregulation of redox homeostasis is implicated in the pathophysiology of many age-related diseases, as well as in the ageing process itself. However, the underlying mechanisms remain largely unclear, which hampers the development of effective therapeutic strategies. To address this challenge, our group leverages the fruit fly Drosophila as a powerful and tractable in vivo system, with its combination of short lifespan and strong evolutionary conservation of central signalling pathways and druggable targets regulating energy metabolism and lifespan.
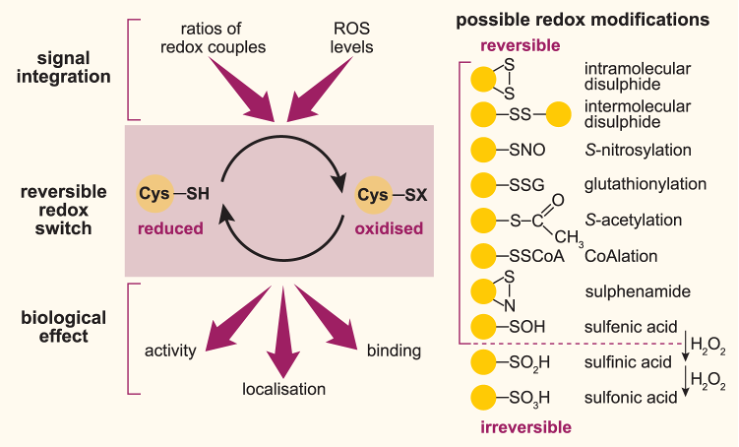
Our research is focused on the following complementary questions:
(1) How does redox signalling regulate metabolic homeostasis and ageing? We aim to identify, characterise and manipulate redox-sensitive targets using a combination of biochemical, proteomic and genetic knock-in approaches, in order to unravel the physiological importance of redox signalling in vivo in the context of metabolic diseases and ageing (e.g. van Leeuwen et al. 2017, Free Rad Biol Med; Cochemé* et al. 2019, BioRxiv).
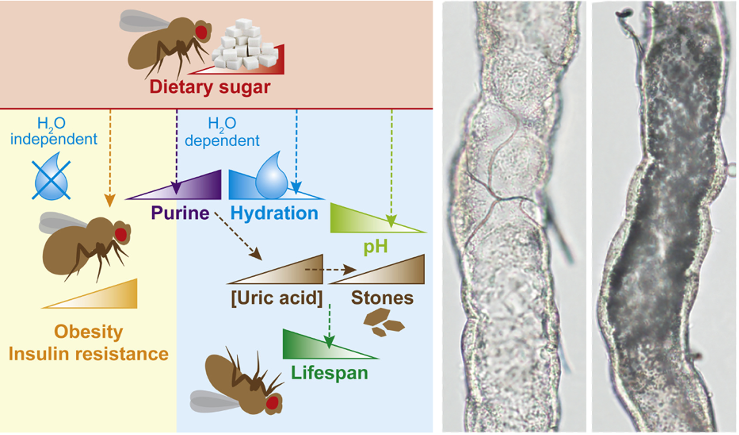
(2) How do diets and drugs impact on survival? We perform dietary and drug interventions shedding light on the complex interplay between redox balance, metabolic dysfunction and ageing. For instance, we recently showed that sugar-induced obesity and insulin resistance can be uncoupled from shortened lifespan, and identified dysregulation of purine catabolism as underlying the pathophysiology of high sugar diets in Drosophila (Figure 2). Furthermore, we extended our study to humans, and found that the association between dietary sugar intake and circulating purines was conserved in a population cohort (van Dam et al. 2020, Cell Metab). We are also interested in exploring evolutionary conserved pharmacological interventions that extend lifespan (e.g. Pryor et al. 2019, Cell).
Ultimately, our goal is to uncover novel pathways and potential therapeutic targets for health and longevity benefits.


dos Santos E, Xie Y, Cao E, Foley A, Taylor ME, Andrew I, Young G, Trevaskis NL, Cochemé HM* (2024) Systemic extracellular acidification is a hallmark of aging BioRxiv 2024.09.24.614672
Lennicke C, Bjedov I, Grönke S, Menger KE, James AM, Foley A, Castillo-Quan JI, Buricova M, Adcott J, Montoya A, Kramer HB, Shliaha PV, Logan A, Cabreiro F, Murphy MP, Partridge L* & Cochemé HM* (2024) Enhancing autophagy by redox regulation extends lifespan in Drosophila BioRxiv 790378 (v2).
dos Santos E & Cochemé HM* (2024) How does a fly die? Insights into ageing from the pathophysiology of Drosophila mortality GeroScience, 46, 4003-4015
dos Santos E & Cochemé HM* (2024) Pharmacology of aging: Drosophila as a tool to validate drug targets for healthy lifespan Aging Biology, 2(1):20240034
Lennicke C & Cochemé HM* (2021) Redox metabolism: ROS as specific molecular regulators of cell signaling and function Molecular Cell 81(18) 3691-3707
Lennicke C & Cochemé HM* (2021) Redox regulation of the insulin signalling pathway Redox Biology 42, 101964
van Dam E, van Leeuwen LAG, dos Santos E, James J, Best L, Lennicke C, Vincent AJ, Marinos G, Foley A, Buricova M, Mokochinski JB, Kramer HB, Lieb W, Laudes M, Franke A, Kaleta C & Cochemé HM* (2020) Sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila Cell Metabolism 31(4), 710-725
Lennicke C & Cochemé HM* (2020) Redox signalling and ageing: insights from Drosophila Biochemical Society Transactions 48(2): 367–377
Pryor R, Norvaisas P, Marinos G, Best L, Thingholm LB, Quintaneiro LM, De Haes W, Esser D, Waschina S, Lujan C, Smith RL, Scott TA, Martinez-Martinez D, Woodward O, Bryson K, Laudes M, Lieb W, Houtkooper RH, Franke A, Temmerman L, Bjedov I, Cochemé HM, Kaleta C* & Cabreiro F* (2019) Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy Cell 178:1299-1312
van Leeuwen LAG, Hinchy EC, Murphy MP, Robb EL & Cochemé HM* (2017). Click-PEGylation – A mobility shift approach to assess the redox state of cysteines in candidate proteins Free Radical Biology & Medicine 108, 374-382
Cochemé HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carré JE, Singer M, Gems D, Hartley RC, Partridge L & Murphy MP (2011) Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix Cell Metabolism 13(3), 340–350