“How does cellular senescence influence cancer and ageing?”
Senescence is a cellular response activated by different stresses such as replicative exhaustion, damage to their DNA, oncogenic activation or inflammation. As part of the senescence programme, cells exit the cell cycle. This stops old, damaged or cancerous cells from replicating incorrectly. Cells undergoing senescence also change their morphology, undergo a significant reorganization of their DNA, rewire their metabolic processes and secretes specific proteins (what is called the Senescence-Associated Secretory Programme, SASP). The SASP allows senescent cells to communicate with their neighbours but also can disrupt tissue homeostasis and contribute to cancer and ageing
Our research is looking to better understand how cells control senescence, and what is the role of senescence in cancer and ageing. We combine high-throughput functional screens (where we can test thousands of interventions) with mechanistic analyses to understand how senescence is regulated and how we can manipulate senescent cells. We use cultured cells and also study how our discoveries apply to models of cancer and ageing.
Eventually, we analyse clinical samples to understand the connection between our observations and the development of age-related human diseases. By better understanding how senescence is initiated and regulated, we can hopefully help improve therapies to treat cancer, many age-related diseases (such as pulmonary fibrosis and metabolic diseases) and aging.
“Senescence influences multiple biological processes including ageing and cancer.”
Cellular senescence is a stress response induced by oncogene activation, chronic inflammation, telomere erosion or DNA damage. Senescent cells activate a dynamic transcriptional programme leading to stable cell cycle exit, chromatin reorganization, metabolic reprogramming and the establishment of a senescence-associated secretory programme (SASP). Acute induction of senescence is a physiological response that limits fibrosis and acts as a potent tumour suppressor mechanism. However, the abnormal accumulation of senescent cells contributes to ageing and many diseases, including cancer. Recently, strategies aimed to selectively eliminate senescent cells (senolytic therapies) have been shown great promise for the treatment of a wide-range of diseases.
The goal of my research program is to elucidate the molecular mechanisms that implement and regulate senescence. We intend to exploit this knowledge to devise novel strategies that target senescence in age-related pathologies, especially cancer. To investigate senescence, we combine high-throughput screens and mechanistic analyses in cell culture, with in vivo analyses in mouse models. We then analyse clinical samples to uncover the relevance of our observations for human disease.
My research programme has two overarching aims: (1) Uncover novel (epi)genetic mechanisms controlling senescence. We will use functional (CRISPR, RNAi and drug) screens, proteomic and genomics to identify trans factors and cis elements that control senescence and elucidate how they function. (2) Investigate how the SASP mediates the effects of senescence. We will perform screens to discover regulators and functions of different subsets of the SASP (e.g. pro- inflammatory or fibrotic) and use novel mouse models to uncover the roles of the SASP in ageing and disease. Connecting both aims, we will search for vulnerabilities of senescence. We are actively collaborating with several companies to pursue novel ways to target senescence cells as a direct result of our research. By better understanding how senescence is regulated and implemented, we expect that our research will inform therapies that harness senescence in cancer and ageing.
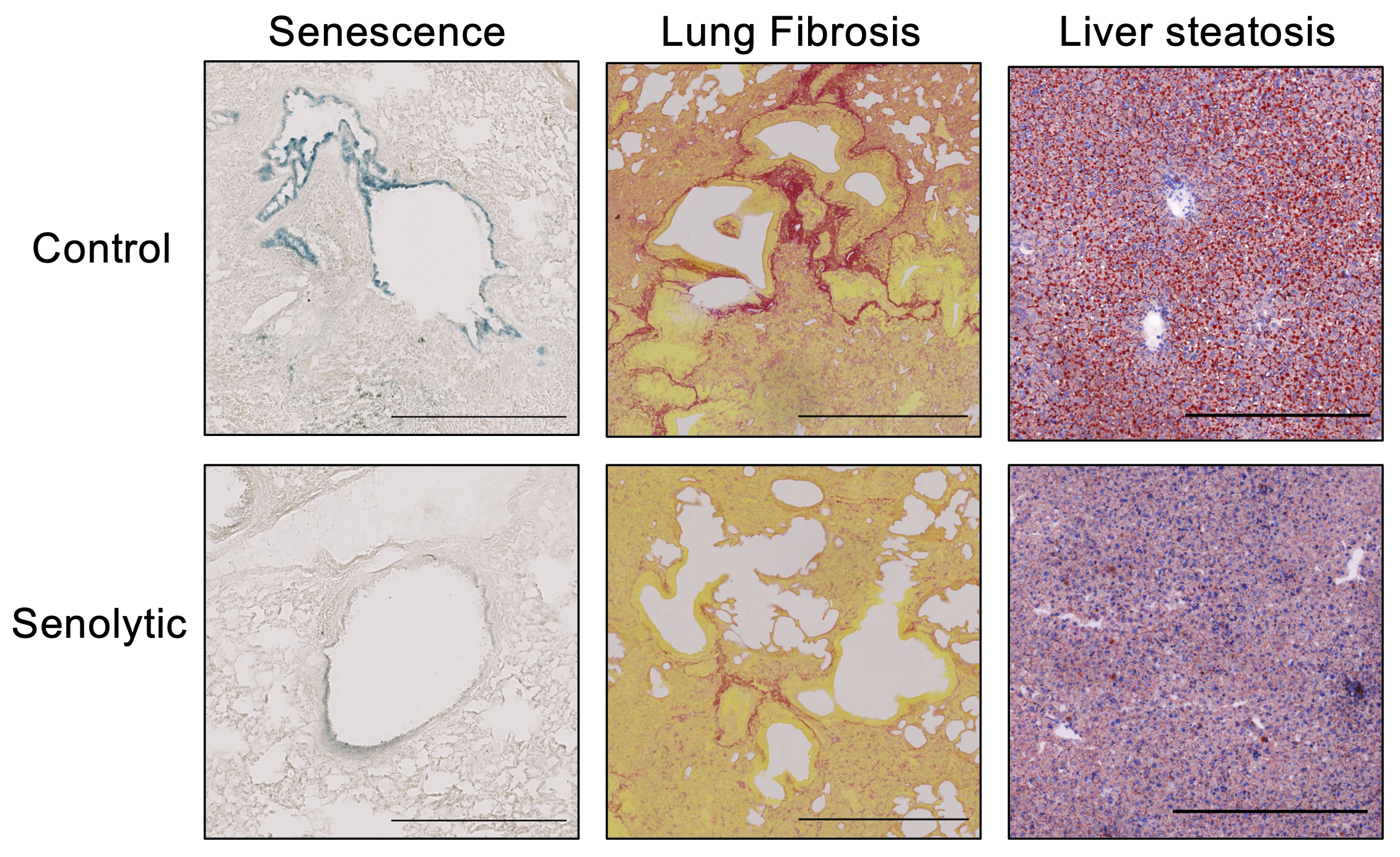
Our research aims to understand how cellular senescence is controlled and can be targeted. Cellular senescence is a stress response that plays important pathophysiological roles. Importantly, senescent cells accumulate and contribute to cancer, ageing, and multiple age-related pathologies.
We take advantage of a combination of approaches, including functional screens to identify novel regulatory mechanisms relevant to senescence. As part of our studies, we are also identifying targets or drugs that can be used to eliminate senescent cells or modulate their effects. Importantly, such drugs (referred to as senotherapies) can be used to treat a broad range of diseases, including idiopathic pulmonary fibrosis, metabolic disease, and cancer.
We have experience in conducting drug and genetic screens and testing modulators of senescence for their effects in ageing, cancer, and liver disease.


Ogrodnik M, Acosta JC, Adams PD, d’Adda di Fagagna F, Baker DJ, Bishop C, Chandra T, Collado M, Gil J, Gorgoulis V, Gruber F, Hara E, Jansen-Dürr P, Jurk D, Khosla S, Kirkland JL, Krizhanovsky V, Minamino T, Niedernhofer L, Passos JF, Ring NAR, Redl H, Robbins P, Rodier F, Scharffetter-Kochanek K, Sedivy J, Sikora E, von Zglinicki T, Yun MH, Grillari J, Demaria M. 2024. MICSE: Minimal Information on Cellular Senescence Experimentation in vivo. Cell, 187:4150-4175.
Duran I, Pombo J, Sun B, Gallage S, Kudo H, McHugh D, Bousset L, Barragan Avila JE, Forlano R, Manousou P, Heikenwalder M, Withers DJ, Vernia S, Goldin RD, and Gil J. 2024. Detection of senescence using machine learning algorithms based on nuclear features. Nat Commun. 15:1041.
McHugh D, Sun B, Hernandez-Gonzalez F, Mellone M, Guiho R, Pombo J, Pietrocola F, Birch J, Kallemeijn W, Khadayate S, Dharmalingam G, Vernia S, Tate E, Martinez-Barbera JP, Withers D, Thomas G, Serrano M and Gil J. 2023. COPI vesicle formation and N-myristoylation are targetable vulnerabilities of senescent cells. Nat Cell Biol. 25(12):1804-1820.
Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A, Moutsopoulos I, Panousopoulos L, Deletic P, Carreno G, Guiho R, Manshaei S, Gonzalez-Meljem JM, Lim HY, Simpson DJ, Birch J, Pallikonda HA, Chandra T, Macias D, Doherty GJ, Rassl DM, Rintoul RC, Signore M, Mohorianu I, Akbar AN, Gil J*, Muñoz-Espín D*, Martinez-Barbera JP*. 2023. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. 41:1242-1260. *Senior authors
Guerrero A, Innes AJ, Roux P-F, Buisman S, Jung J, Ortet L, Robinson L, Ausema A, Perdiguero E, Aarts M, Martin N, Muñoz-Canoves P, de Haan G, Bischof O and Gil J. 2022. 3-deazaadenosine (3DA) alleviates senescence to promote cellular fitness and therapy efficiency. Nat Aging, 2:851-866.
Innes AJ, Bin Sun B, Wagner V, Brookes S, McHugh D, Pombo J, Porreca RM, Dharmalingam G, Vernia S, Zuber J, Vannier J-B, García-Escudero R and Gil J. 2021. XPO7 is a tumor suppressor regulating p21CIP1-dependent senescence. Genes Dev, 35, 379-391.
Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, Wolter K, Pombo J, Irvine E, Innes AJ, Birch J, Glegola J, Manshaei S, Heide D, Dharmalingam G, Harbig J, Olona A, Behmoaras J, Dauch D, Uren AG, Zender L, Vernia S, Martínez-Barbera JP, Heikenwalder M, Withers DJ and Gil J. 2019. Cardiac glycosides are broad-spectrum senolytics. Nature Metabolism, doi:10.1038/s42255-019-0122-z
Gorgoulis V, Adams P, Alimonti A, Bennett D, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier A, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M and Demaria M. 2019. Cellular senescence: a path forward. Cell, 179, 813-827.
Georgilis A, Klotz S, Hanley CJ, Weirich B, Morancho B, Herranz N, Leote AC, Carroll T, Dharmalingam G, Wee KB, Mellone M, Guccione E, Arribas J, Barbosa-Morais NL, Heikenwalder M, Thomas GJ, Zender L and Gil J. 2018. PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell, 34, 85-102.
Acosta JC, Banito A, Wuestefeld W, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Mindaugas A, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman G, Longerich T, Sansom OJ, Benitah SA, Zender L and Gil J. 2013. A complex secretory response orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology 15, 978-990.
Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert, A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E and Gil J. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence, Cell 133, 1006–1018.