“How does diet affect liver function?”
Our work focuses on better understanding the biochemistry of metabolism in the body, and how these alter in those with conditions such as obesity and diabetes. Following a high-fat diet can induce insulin resistance, lead to type 2 diabetes and even some cancers.
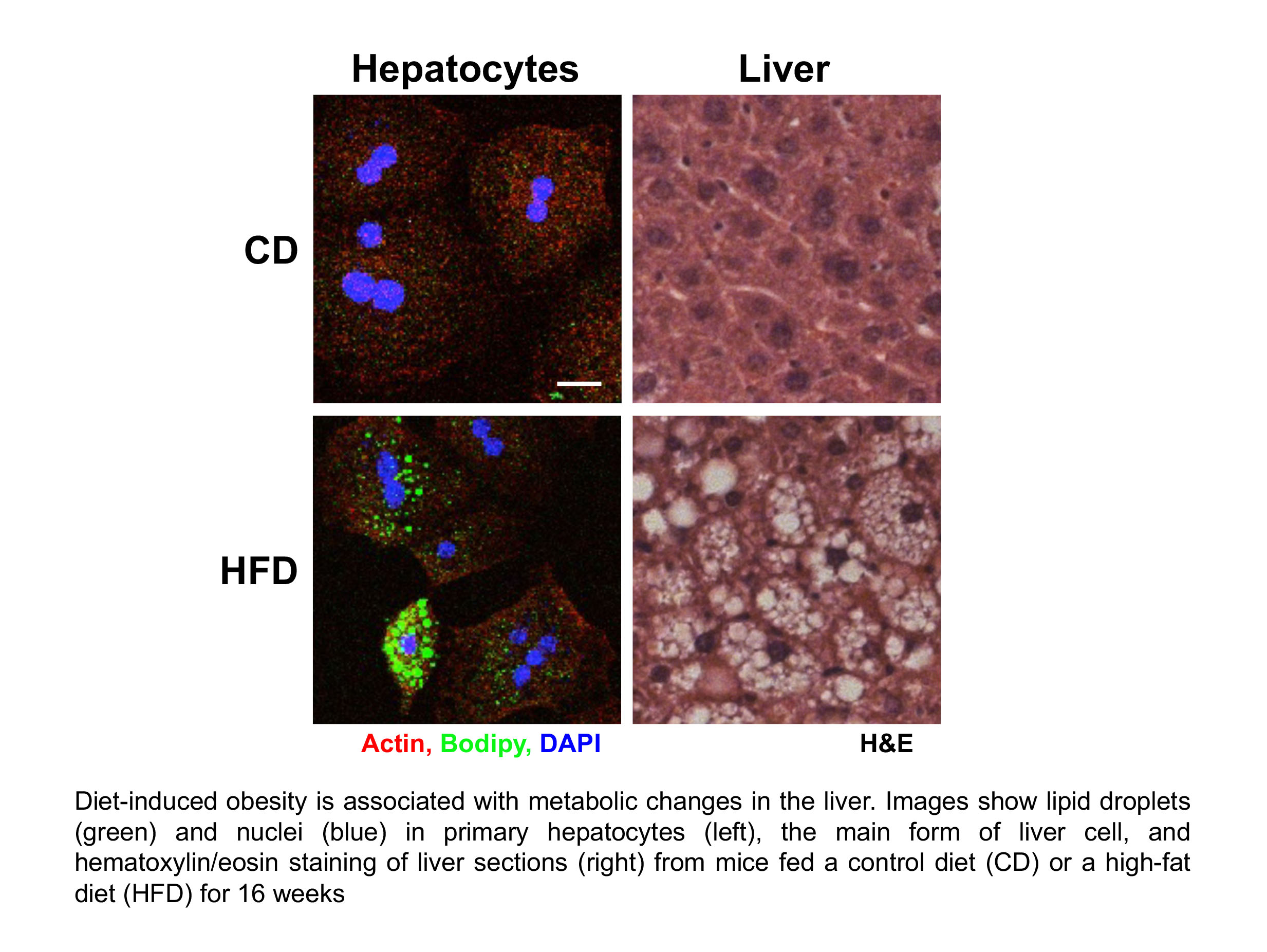
The liver is critical in regulating our metabolism and is sensitive to dietary changes. It helps maintain normal levels of glucose, although struggles to do so if under strain from a high-fat diet. Eating healthily can lower liver fat levels and reduce the expressions of stress and inflammatory genes that can contribute to aging.
We hope to characterise the molecular mechanisms that drive obesity-related liver disease as it develops. We are particularly interested in how the expression of certain genes change as liver diseases progress, and how this could be modulated.
We use computational and experimental approaches to identify and better understand the metabolic regulators. We use mouse models to find out more about the impact diet can have on liver function, and how their cellular metabolism changes over time.
We know that diet plays a crucial role in shaping human health and disease. A better understanding of how diseases develop due to over consumption may pave the way for the development of disruptive or therapeutic treatments, or approaches to stop the diseases developing in the first place.
“We intend to characterise the molecular mechanisms driving metabolic homeostasis in health and in metabolic pathologies such as diabetes and obesity.”
The rising prevalence of metabolic pathologies such as obesity, type 2 diabetes (T2D) or non-alcoholic fatty liver disease (NAFLD) constitutes a major health problem.
We use both computational and experimental approaches to identify and characterize new metabolic regulators relevant for human metabolic pathologies to uncover novel targets for the design of improved diagnostic and therapeutic strategies.
Our short-term goal is to characterize the molecular mechanisms driving obesity-associated liver disease at different stages (insulin resistance, NAFLD, nonalcoholic steatohepatitis, fibrosis or hepatocellular carcinoma) with an emphasis on the role of pre-RNA splicing and alternative splicing in this process.

Kokkinou M, Irvine EE, Bonsall DR, Natesan S, Wells LA, Smith M, Glegola J, Paul EJ, Tossell K, Veronese M, Khadayate S, Dedic N, Hopkins SC, Ungless MA, Withers DJ*, Howes OD*. (2020). Reproducing the dopamine pathophysiology of schizophrenia and approaches to ameliorate it: a translational imaging study with ketamine. Molecular Psychiatry doi: 10.1038/s41380-020-0740-6 *joint communicating authors.
Smith MA, Choudhury AI, Glegola JA, Viskaitis P, Irvine EE, Custodio de Campos Silva PC, Khadayate S, Zeilhofer HU, Withers, DJ. (2020). Extrahypothalamic GABAergic nociceptin-expressing neurons regulate AgRP neuron activity to control feeding behaviour. The Journal of Clinical Investigation 130, 126-142.
Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, Wolter K, Pombo J, Irvine EE, Innes AJ, Birch J, Glegola J, Manshaei S, Heide D, Dharmalingam G, Harbig J, Olona A, Behmoaras J, Dauch D, Uren AG, Zender L, Vernia S, Martínez-Barbera JP, Heikenwalder M, Withers DJ, Gil J. (2019). Cardiac glycosides are broad-spectrum senolytics. Nature Metabolism 1, 1074-1088.
Rached M-T, Millership SJ, Pedroni SMA, Choudhury AI, Costa ASH, Hardy DG, Glegola JA, Irvine EE, Selman C, Woodberry MC, Yadav VK, Khadayte S, Vidal-Puig A, Virtue S, Frezza C, Withers DJ. (2019). Deletion of myeloid IRS2 enhances adipose tissue sympathetic nerve function and limits obesity. Molecular Metabolism 20, 38-50.
Smith MA, Katsouri L, Virtue S, Chouhury AI, Vidal-Puig A, Ashford MLJ, Wither DJ. (2018). Calcium channel Ca(v)2.3 subunits regulate hepatic glucose production by modulating leptin-induced excitation of arcuate pro-opiomelanocortin neurons. Cell Reports 25(2), 278-287.
Millership SJ, Da Silva Xavier G, Choudhury AI, Bertazzo S, Chabosseau P Pedroni SM, Irvine EE, Montoya A, Faull P, Taylor WR, Kerr-Conte J, Pattou F, Ferrer J, Christian M, John RM, Latreille M, Liu M1 Rutter GA, Scott J, Withers DJ. (2018). Neuronatin regulates pancreatic β cell insulin content and secretion. The Journal of Clinical Investigation 1, 128(8), 3369-3381. doi: 10.1172/JCI120115.
Viskaitis P, Irvine EE, Smith MA, Choudhury AI, Alvarez-Curto E, Glegola JA, Hardy DG, Pedroni SMA, Paiva Pessoa MR, Fernando ABP, Katsouri L, Sardini A, Ungless MA, Milligan G, Withers DJ. (2017). Modulation of SF1 Neuron Activity Coordinately Regulates Both Feeding Behavior and Associated Emotional States. Cell Reports 21(12), 3559-3572.
Smith MA, Katsouri L, Irvine EE, Hankir MK, Pedroni SM, Voshol PJ, Gordon MW, Choudhury AI, Woods A, Vidal-Puig A, Carling D, Withers DJ. (2015). Ribosomal S6K1 in POMC and AgRP Neurons Regulates Glucose Homeostasis but Not Feeding Behavior in Mice. Cell Reports 11(3), 335-343.
Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. (2009). Ribosomal protein s6 kinase 1 signaling regulates mammalian life span. Science 326(5949), 140–144.