By Sophie Arthur
November 14, 2019
Time to read: 6 minutes
More people than ever have diabetes, and more people than ever are at risk of developing diabetes. If nothing changes, more than 5 million people will have diabetes in the UK by 2025. For World Diabetes Day, we sat down with Mathieu Latreille, Head of the Cell Identity and Metabolism group at the LMS, and one of his PhD students, Eva Kane, to talk about their motivations, the issues they are aiming to solve and how their research may provide a new solution for diabetics.

Pancreatic beta cells are responsible for insulin release and thus are crucial for maintaining balance of our blood glucose levels. Diabetes is a metabolic disease that occurs when our blood glucose levels are too high due to an insulin deficiency, or long periods of insulin resistance. There are many types of diabetes, but the two most common and well-known ones are type I and type II. Both Mathieu and Eva are interested in diabetes research as they are fascinated by the biology behind insulin-producing beta cells, particularly by the complex development of beta cells in the embryo, as well as their ability to change their identity in diabetes. In this latter process, beta cells enter an intermediate stage better known as “dedifferentiation” enabling them to adopt a completely different identity and function. Mostly excitingly, these cells display the ability to gain back their original identity. Their research is looking at how the processes underlying the development and change in beta cell identity could be combined to come up with a new and effective therapeutic outcome for diabetic patients.
The current diabetes landscape
Diabetes has long been attributed to a decrease in beta cell function and mass, but with the recent recognition that beta cells can lose their identity, it could also contribute to a decline in insulin production. However, current treatments are looking at ways to increase beta cell mass or increase the rate at which they proliferate. One way is through islet transplantation from organ donors. While this approach could cure and produce a good outcome for those with very treatment resistance diabetes, there are many disadvantages also. Over the past 20 years, less than 1000 of these transplants have taken place because there are few donors that are suitable, the logistics are difficult and the procedure is not very efficient. Other ways as a means to increase beta cell mass is using embryonic stem cells and directing them along a very complex path to help them become beta cells mimicking the process that happens during development. However, this process has its disadvantages too. The current protocol to direct these stem cells and differentiate them into beta cells is a very complex network that still isn’t completely understood, cells are lost at every stage of this process and even at the final cell preparation step, many of the beta cells are still not functionally mature. So, you would be transplanting very few cells and many of which are not ready to produce insulin.
But the field is shifting even more as recently researchers have been looking at encapsulating mature beta cells or stem cell-derived beta cells in mini organs protecting them from immune attack – similarly to how an organ might get rejected in a transplant – and that mimic their natural environment, or looking at converting another pancreatic cell type into beta cells. But Mathieu and Eva are looking at yet another potential approach that could help in the future with diabetes treatments. They are investigating the possibility of using agents that inhibit the function of microRNAs to stimulate beta cell regeneration.
MicroRNAs for diabetes treatment
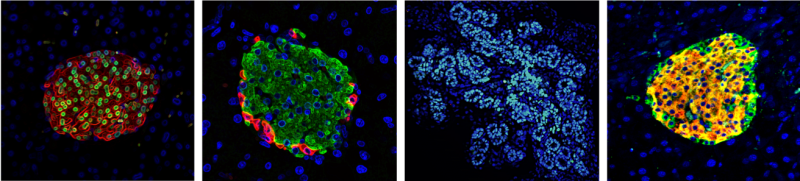
MicroRNAs are a group of small non-coding RNAs that help regulate gene expression. They bind to their target messenger RNA (mRNA) molecules and reduce gene expression by stopping the mRNA being translated into a protein. MicroRNAs are well established as potential targets of therapeutic agents which can help with diseases such as hepatitis C, other metabolic diseases and cancer. This supports the idea then that a microRNA could be a target for a diabetes treatment, especially as great strides in the understanding of how microRNAs impact beta cell identity have been made recently.
Mathieu‘s lab is particularly interested in a subset of microRNAs which they found to trigger loss of beta cell identity when the microRNA was over expressed in beta cells. It has also been shown that if you inhibit the function of these microRNAs, the effects can be reversed, as blood glucose levels were rescued and dedifferentiated cells regain their beta cell identity when this was tested in genetically modified mice. This provides a proof of principle for improving pancreatic beta cell function in diabetes following inhibition of some microRNAs.
There are some advantages for targeting microRNAs in the treatment of diabetes. The first being that microRNAs have very tissue specific expression patterns which means they are only expressed in certain cells of the body. Eva’s data revealed that miR-7, for example, is crucial during the development of insulin-producing cells in the mouse embryo. Moreover, microRNAs have the ability to target many genes simultaneously, which control commitment of immature cells into beta cells. Moreover, there is no need to perform a drug screen to determine what molecules can target the microRNAs as there are already a specific class of molecules that do that called antisense microRNAs. In fact, many biopharmaceutical companies are currently developing anti-microRNAs and these have already been used in different settings to modulate and delay disease appearance. Many anti-microRNAs are currently being assessed in phase 2 clinical trials and so are very likely to be approved. A final key advantage is that this type of treatment could help both type I and type II diabetics.
Mathieu discussed his ambitions for the future of these diabetes treatments with us:
“Translating our finding into a therapy is my group’s ultimate goal. We have found a target that I am really confident in and that we could get something out of it down the line that is beneficial to diabetics and this is what is really driving me right now. From Eva’s project, we have really promising data in mice, so the next big hurdle is to get that into humans, and develop antisense microRNAs that can specifically target beta cells.”
Earlier this year, Eva gave a fantastic talk at one of our Pint of Science events about ‘building beta cells to cure diabtetes’ which you can watch here to find out more about this research: