By Helen Figueira
November 29, 2017
Time to read: 3 minutes
Exciting new research published this week from the group of Jesus Gil has identified novel roles for some well-known genes in regulating senescence during cell reprogramming. The work reveals new links between senescence and induction of reprogramming– in particular a role for the well characterised regulatory kinase mTOR, which is already considered a potential therapeutic target for cancer, neurological and metabolic diseases.
The Gil group’s primary research focus is to understand the regulation of senescence in aging and cancer. Senescence involves the loss of a cell’s ability to divide and grow, and accumulation of senescent cells is now known to be a factor in ageing and in many age-related diseases.
Gil’s latest work sought to understand the peculiarities of the senescence program that occurs during cellular reprogramming. The ability to reprogramme somatic cells – cells that have differentiated and ceased to divide – into pluripotent stem cells (iPSCs) is a key process necessary for developing novel regenerative medicines. Senescence is a major limiting factor to the efficiency of cellular reprogramming and a lot remains to be understood about how the pathways involved in inducing senescence or pluripotency in this context are controlled. “If we get a better understanding of how senescence influences reprogramming we could improve the processes to generate stem cells that could be of use for regenerative medicine” says Gil.
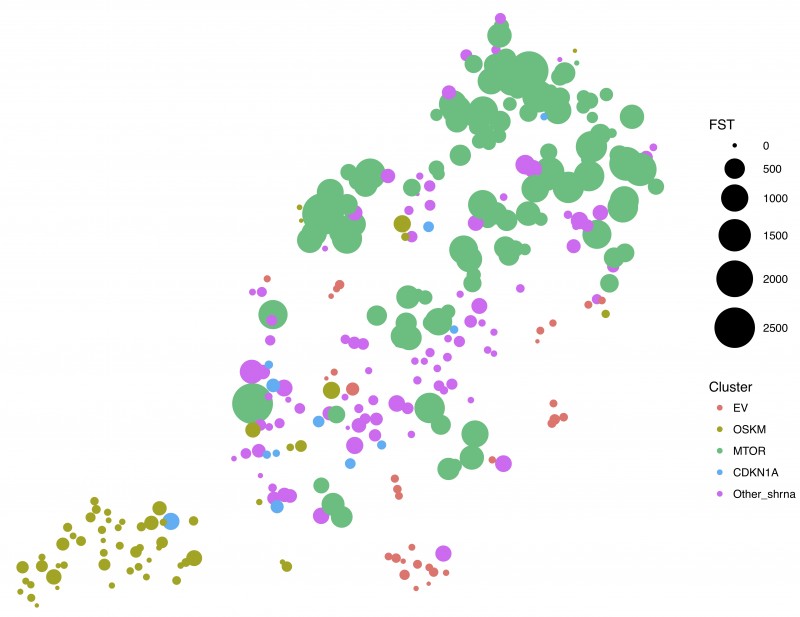
Reprogramming can be promoted by expressing pluripotency-associated factors (OCT4, SOX2, KLF4 and cMYC aka OSKM). Using a combination of shRNA screening and single-cell RNA sequencing (scRNA-Seq) Gil’s group found that despite some similarities to other types of senescence such as that induced by oncogenes, OSKM-induced senescence is a divergent process. shRNA screening of 58,000 genes identified four candidates (CDKN1A, MYOT, MTOR and UBE2E1) whose depletion prevented OSKM-induced senescence.
MTOR is a cellular kinase with well characterised functions in autophagy, cell growth, proliferation, motility and survival. MTOR had been shown to influence reprogramming but it was unclear how. Previous work trying to understand how mTOR controls reprogramming had focused on the connection between mTOR and autophagy. This work highlights senescence as an additional way by which mTOR can regulate reprogramming. Using single cell analysis, Gil’s group went on to show that depletion of mTOR affected different aspects of senescence, controlling cell cycle (via the cyclin dependent kinase inhibitor p21) and the secretion of multiple factors such as IL6 or TGF-b. Interestingly, while re-entering into cell cycle increases reprogramming, inhibiting the ‘senescence secretome’ has the opposite effect. Therefore, this suggests an explanation for the contradictory effects that mTOR inhibition has over reprogramming.
“It was known that mTOR is important for senescence and reprogramming, but this is the first time the two processes have been brought together” says Gil. “It’s also exciting how we were able to accelerate the characterisation of candidates identified in functional screens by taking advantage of single cell RNA-Seq”
You can read the full paper here http://genesdev.cshlp.org/content/31/20/2085.full