By Sophie Arthur
October 11, 2019
Time to read: 5 minutes
Obesity is a disease that is still very misunderstood. So, for World Obesity Day today we asked post-doc Alice Pollard to share a little more light on the condition, help us realise how cool fat cells really are as well as some of her amazing research in the fight against obesity.

Obesity, a complex disease that is influenced by many different factors, is characterised by an increase in fat mass, or adipose tissue, accumulated when energy intake exceeds energy usage. However, we now recognise that the fat we cannot see, that which surrounds our internal organs and accumulates in places it should not, drives many aspects of this condition. These organs are ill equipped to deal with an increase in fat, or lipid ,accumulation, and if left untreated, can lead to life threatening conditions referred to as the metabolic syndrome, including Type II diabetes, atherosclerosis, non-alcoholic steatohepatitis and cancer.
Fat: friend or foe?
Adipose tissue is responsible for buffering nutrient excess, producing hormones, responding to the energy demands of other tissues. In times of hardship, it is an effective insulator and possesses the ability to generate heat, making it a true master regulator of whole organism energy homeostasis, or balance.
Historically, adipose tissue comes in two flavours: brown and white. Brown adipose tissue, or BAT, shares a cell ancestor (common stem cell) with skeletal muscle, and the two have much in common. Both contain many mitochondria (small membrane bound organelles responsible for the production of the majority of the cells energy), and produce heat through a process known as non-shivering thermogenesis (NST). In contrast, the white adipose tissue (WAT) is largely responsible for lipid storage, insulation and hormone production. Recent studies have put this brown and white view of adipose tissue to the test, however, beginning with the discovery of an intermediate cell type, termed ‘beige’ or ‘brite’. These cells are primarily induced by exposure to cold, and exhibit several key features of true brown adipocytes, namely their ability to generate heat through the expression of a specialised protein: UCP1.
In BAT, UCP1 sits in the membrane of the mitochondria and is responsible for ‘short-circuiting’ the energy-generating processes occurring in favour of heat generation. In small mammals, such as mice and rats, UCP1 is indispensable for short-term cold exposure. In humans, BAT is most abundant in newborn babies, where it is essential for warmth in the absence of alternative insulation. Both mass and function then decrease over time, in part due to its redundancy given our thermoneutral environment, and even more so in cases of obesity, where it is largely engulfed by white adipose. Many efforts have been made to increase functional BAT and thus promote calorie burning through non-shivering thermogenesis in humans, for the treatment of metabolic disorders. However, these treatments are ineffective with additional effects on heart rate and core temperature rendering them unsafe.
When fat acts more like muscle
Our laboratory at the MRC LMS studies a protein known as AMP-activated protein kinase (AMPK). In response to energy deprivation, such as exercise, this enzyme is responsible for the co-ordination of both rapid and long-term solutions to restore energy balance in individual cells and across the whole organism. When active, AMPK achieves this by shutting down processes that consume energy (ATP) and promotes those that regenerate it. Many studies have linked AMPK activation with an increase in BAT health, protection from lipid accumulation and an increase in energy expenditure, making it a promising target for the treatment of obesity and the metabolic syndrome.
My research at AstraZeneca, in collaboration with the MRC, focuses on the effects of activating AMPK both genetically and with small molecules. We have shown that activating AMPK protects from diet-induced obesity in mice, through an increase in whole organism energy expenditure. In this model, we observed a striking ‘browning’ of white adipose tissue, with one surprising omission: UCP1. Instead, these cells expressed proteins used by their close cousins, skeletal muscle cells, to provide an alternate method for producing heat in response to nutrient excess. Termed calcium futile cycling, this process is used preferentially by several species, including birds and some fish to maintain high body temperatures without sacrificing ATP production. Though well characterised muscle cells, the role of this pathway in other cells requires further investigation.
Using a technique called RNA sequencing to read the genetic signature of these cells, we discovered that they share many features of true brown adipocytes and express genes that link them to a cell lineage shared with muscle. This switch in identity then allows them to tap into calcium futile cycling to create a sustainable form of thermogenesis, bypassing UCP1. Due to their unique, muscle-like activity, we termed these cells SMART (Skeletal Muscle-like AMPK Reprogrammed Thermogenic) adipocytes.
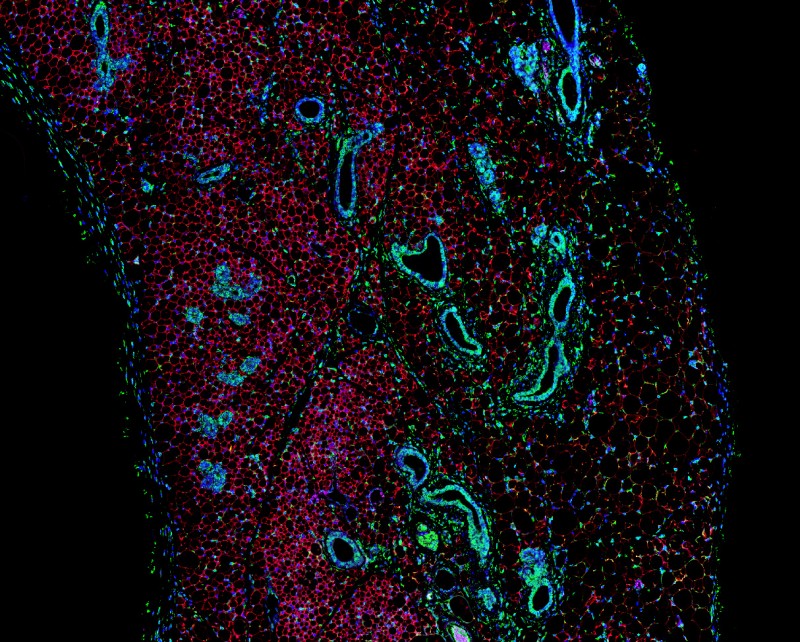
These findings highlight the need to understand further the decisions cells make in choosing their fate, and how changes in metabolism can promote alterations in lineage determination. Using a range of exciting techniques including single cell RNA sequencing and whole tissue imaging, we are exploring the development of this novel cell population, how they regulate energy expenditure and their potential as a therapy for the treatment of metabolic disorders. It is our hope that in future, fat will be viewed as a friend, not a foe, in the fight against obesity.