Decoding Obesity: Unraveling the DNA Connection
Home > News > Decoding Obesity: Unraveling the DNA Connection
New research has identified over 800 DNA switches in human fat cells in individuals with extreme obesity. Many of these switches appear to be ‘crucial determinants’ of human obesity and its harmful health consequences. Manipulating these switches could be an effective new strategy for obesity treatment.
By LMS Staff Member
June 29, 2023
Time to read: 3 minutes

Obesity rates have quadrupled worldwide since the 1980s, making it one of the biggest health concerns of our time. This complex disease is closely linked to the leading causes of death worldwide, including heart disease and stroke resulting in the loss of approximately 22.9 million lives each year, about the population of New York City.
Variations in our genetic code alone do not explain the sharp increase in obesity over recent decades. We now understand that interactions between our genes and our environment can cause modifications to DNA. These modifications affect how our body interprets signals to switch genes on or off and is a growing field of study known as epigenetics. What makes these epigenetic switches particularly promising is their reversibility, opening the door to potential therapeutic interventions.
One well-studied epigenetic switch, DNA methylation (the addition to DNA of a ‘methyl’ group comprising one carbon and 3 hydrogen atoms), has received considerable attention. DNA methylation can switch genes on and off, however, previous attempts to establish whether DNA methylation has a causal role in human obesity have proved inconclusive.
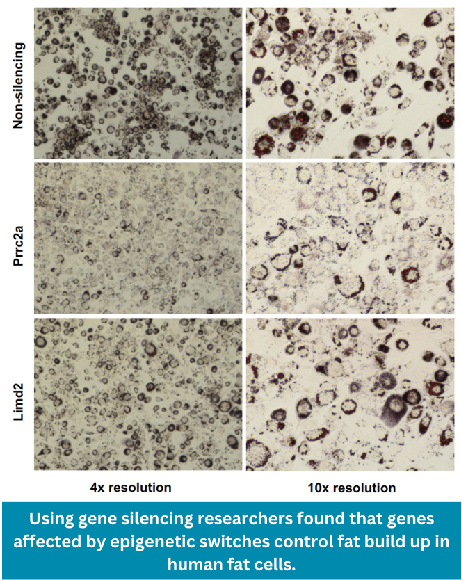
Now a team, led by the MRC LMS Genomics of Obesity Group, conducted a study comparing the fat cells of individuals with extreme obesity to those with a healthy body weight. This research, published in Nature Comms, discovered a staggering 864 DNA methylation sites that were switched on or off in the fat cells of people with extreme obesity. They also found more than 500 genes that were changed by the DNA methylation switches in people with obesity but not those with a healthy body weight.
‘This research has the potential to revolutionise our understanding and treatment of obesity. We are getting closer to personalised and effective treatments as we unravel the complex connections between our DNA and our health.’
– Damir Baranašić, researcher in the MRC LMS Computational Regulatory Genomics group
Using cutting-edge computational techniques with the MRC LMS Computational Regulatory Genomics Group the researchers showed that a large proportion of these DNA methylation switches were critical determinants of obesity and its main complication, type 2 diabetes. They further revealed the significance and mechanisms of these DNA methylation sites using the gene editing technology CRISPR in fat cells.
Damir Baranašić, researcher in the MRC LMS Computational Regulatory Genomics group, stated,
“This work, led by Will Scott, is a testament to the power of collaborative efforts in our Institute. This research has the potential to revolutionise our understanding and treatment of obesity. We are getting closer to personalised and effective treatments as we unravel the complex connections between our DNA and our health.”
There is an urgent need for effective solutions for the growing rates of obesity. This innovative study provides crucial evidence to advance the development of personalised medicine for individuals living with obesity. Will Scott, the study lead explained,
“We have shown that epigenetic switches on our DNA do contribute to susceptibility to obesity and diabetes. This is important because it opens up a range of possibilities for modifying harmful DNA switches to treat people with these conditions.”
Moving forward, the MRC LMS aims to continue this vital research, to tackle the challenge of obesity and improve human health.
Further information and support on Obesity





