By Helen Figueira
May 13, 2012
Time to read: 3 minutes
 First litter of GFP transgenic rats generated
First litter of GFP transgenic rats generated
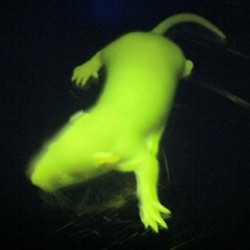
Glow-in-the-dark rats might not look like a biomedical breakthrough, but there’s more to these radiant rodents than meets the eye. They represent the first successful attempt by an MRC team to alter the genetic make-up of a rat. By inserting a gene that codes for Green Fluorescent Protein (GFP) into rat embryos, the scientists have been able to show in spectacular fashion that genetic modification in this species – something that has long eluded scientists – is possible.
The litter of rat pups marks a great achievement for the team from the Transgenics and Embryonic Stem Cell Laboratory and the Physiological Genomics and Medicine group. The process of inserting a new gene into the genome of the animal is far from straightforward. A sliver of DNA (a circular molecule of DNA called a plasmid) is injected directly into the rat embryo. This plasmid includes the GFP gene and a strong promoter – a chunk of DNA that drives expression of the gene it is adjacent to. Once this DNA is absorbed into the genome of the embryo, every cell in the resulting rat expresses the gene, making each cell glow green under blue light.
This technique has long been used to experiment with the genetics of mice, but its application in rats has proved more tricky. “It’s very difficult to do this with rats,” say the scientists. “But rats are physiologically closer to humans than mice are. You can mimic human phenotypes or pathologies, such as hypertension or insulin resistance, much more easily in a rat than a mouse.”
“This was a proof of concept,” the team explains. “In order to optimise the whole technique, we needed a simple construct, that was very easy to identify.” The fluorescent gene provided just that. This first litter of rats come from a line of rats known as Sprague Dawley. These are normal, healthy rats, with conventional genetic backgrounds (that is, they have not been inbred or genetically modified).
The challenge now is to repeat the feat using embryos derived from particular inbred rat strains, such as Wistar Kyoto (WKY), which they have already found success with. Such strains are of significant interest because their entire genetic background is known, making changes to the genome easier to identify and analyse. Embryos from these strains have, however, proven more difficult to manipulate.
Recent advances in zinc finger nuclease technology will make it possible to specifically alter select genes in these lines. This technique allows DNA-cutting enzymes to target very specific regions of DNA, meaning that unique genes can be silenced. This means the researchers can dissect the exact role of specific genes.
The first plans for this technique include using the WKY strain of rat, and another that spontaneously develops hypertension (the “SHR” strain) to look at pathologies associated with insulin resistance, hypertension, and glomerulonephritis. But as the technique is perfected, this could present a vital new tool and animal model for the full spectrum of human disease. The future is very bright indeed.
–AL