By Sophie Arthur
March 18, 2020
Time to read: 4 minutes
Fruit flies have been used as a model organism and experimental system in biomedical research for hundreds of years. In fact, their use has been responsible for some of the most significant advances in the field of medicine. But what if understanding the biology of the fruit fly itself could help us come up with interventions that could directly affect human health? For example, there is a need to find alternative measures that can held the spread of malaria and other insect-transmissible diseases. The latest discoveries and advances published in Nature from our Gut Signalling and Metabolism group have shed some light on a potential new route.
It all started with nutrient sensors
The ability for cells in the gut to sense nutrients is essential to maintain homeostasis – the balance of internal, physical, and chemical conditions maintained by living systems – and their ability to adapt to a changing environment. These nutrient sensors are most commonly found in the gut lining, specifically in the enteroendocrine cells. These are specialised cells that produce and release hormones in response to changes in the external environment. However, our colleagues were curious about enterocytes – which play more of a role in digestion and absorption of nutrients – and whether they have any ability to sense nutrients themselves.
Using Drosophila melanogaster as an experimental system, a genetic screen was performed and investigated whether over 100 different proteins were involved in nutrient sensing. This screen identified a receptor that not only had a role in sustaining food intake but also developmental growth, as when this protein was removed, there was a significant delay in development. This receptor was named Hodor; an abbreviation of ‘hold on, don’t rush’ as a result of the observed developmental delay.
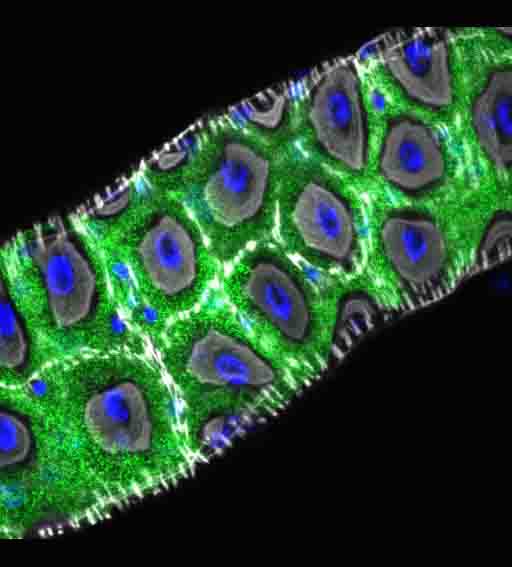
In a large collaborative effort involving scientists from Harvard University, Wellcome Sanger Institute, IGFL Lyon, Max Planck Institute of Molecular Cell Biology and Genetics, University of Manchester, Imperial College London and the Molecular Systems group here at the MRC LMS, the team established that Hodor is a zinc-sensing receptor that is expressed in enterocytes found in the gut lining. It uses the metal to facilitate the transport of the element chloride into and out of cells which enables the initiation of further pathways that sustain food intake and developmental growth. Metals have received little attention when it comes to investigating their role in development. These findings that show metals, particularly zinc, and their sensors are not just essential for food intake and development, but they are instructive too.
From flies to mosquitos
As Hodor-like proteins are only found in insects, these receptors may also be highly valuable for the control of disease-transmitting insect populations, such as mosquitos. If Hodor has the same role within food intake and development in mosquitos as it does in flies, then there is huge potential for healthcare applications.
To explore this possibility, the Gut Signalling and Metabolism team created mosquito mutants that were lacking in the expression of Hodor. Deleting this gene in Anopheles mosquitos was fatal: mosquitoes did not make it past their larval stage. The fact that Hodor is a receptor that is expressed in the gut lining is more attractive as a potential way to help curb mosquito populations. It overcomes any physical barriers a drug may have of reaching its target, as any drug blocking the action of Hodor could be ingested easily and act to curb a mosquito population. As Hodor is an insect-specific gene, any potential drugs that target the sensor may be harmless to humans also to overcome another challenge with interventions to control disease-transmitting insects. But it does again emphasise the value of investigating insect specific biology as it is not only human research that can lead to treatments and interventions.
Professor Irene Miguel-Aliaga, Head of the Gut Signalling and Metabolism group at the MRC LMS, discussed the next steps for this research:
“
Firstly, we want to understand more about how this intestinal sensor affects food intake. Maybe there is a signal from the intestine to the brain to control this, but we don’t know what that signal involves and we want to explore that further. Secondly, we want to investigate what exactly Hodor is doing in the enterocytes where it is expressed; this may have something to do subcellular structures called lysosomes”.
‘An intestinal zinc sensor regulates food intake and developmental growth’ was published on 18 March in Nature. Read the article here.