By Sophie Arthur
October 12, 2018
Time to read: 5 minutes
The Synaptic Plasticity and Repair group at Imperial College London Institute of Clinical Sciences, headed by Dr Vincenzo De Paola, study the connections between nerve cells (neurons) in the brain. They investigate how the connections (synapses) between neurons are formed, maintained and lost. An area very important in understanding the functioning of the brain both in individuals who are healthy and those who have neurodevelopmental and neurodegenerative conditions.
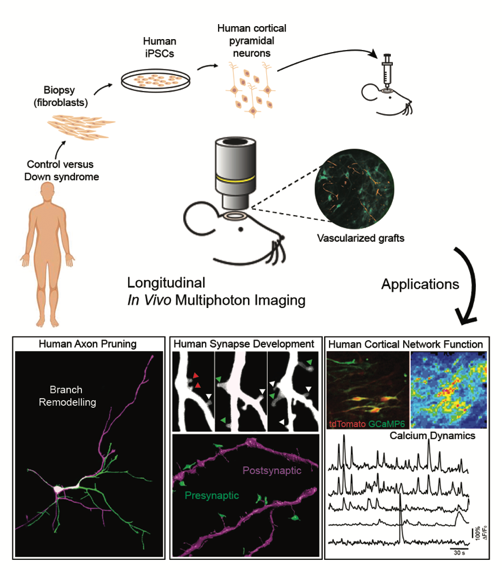
Their recent study, published in Science on 11 October, shows a new model for investigating and monitoring in real time human neuron development and function in a living brain. The research, in collaboration with a team from Cambridge University, displays differences between neuron and synapse development in healthy individuals and those with the neurodevelopmental condition Down syndrome.
The loss of synapses is tightly linked to cognitive impairment in patients. In neurodegenerative disorders, such as Alzheimer’s, the loss of synapses at earlier stages is one of the first steps preceding the death of the whole neuron. It is therefore of great importance to understand how synapses work and are lost.
Synapses are so small that they cannot be studied within the human brain with the current imaging methods available. Traditionally, researchers would use model organisms such as mice, flies, fish and worms, to investigate neuron and synapse development. This, however, raises the question of how well this relates to understanding the neurons in humans. The study of neurons in these model organisms, while still essential, is known to not always translate to humans and if this is the case, observations and predictions made within these traditional types of study may lose some of their impact.
The inability to study human neurons in vivo has been an important problem that Dr De Paola and his team, as well as other researchers around the world, have been thinking about for a long time. Following the breakthrough of induced pluripotent stem cells (iPSCs) a few years ago, the study of human neurons in vivo was able to advance.
In this pioneering study, human neurons were created by reverse-engineering skin cells from two individuals with Down syndrome by co-first author Dr Manuel Peter and colleagues in Prof Livesey’s group at the Gurdon Institute. The cells were implanted in the brains of mice by co-first authors Dr Raquel Real and Dr Antonio Trabalza of Dr De Paola’s team, creating a model where the development of the neurons and synapses can be observed in real time. There were three key findings of the study: the first was that when the human neurons were transplanted connections developed with similar timing and extent to the human foetal cortex. The second, that transplanted human neurons function as expected, with spontaneously synchronised activity similar to the human foetal cortex. The third, that spontaneous neural activity is markedly reduced in individuals with Down syndrome.
The third finding, which shows clear differences in the activity of the neurons and synapses in healthy individuals and those with Down syndrome, is a very important one. The different synchronised activity may have to do with cognitive function. The team will be exploring this next, as they believe there are candidate molecules involved in the markedly reduced activity and are eager to investigate them.
These live imaging experiments to study human synaptic development and function in complex genetic conditions such as Down syndrome had never been done before and the results went beyond any expectations the group had, Dr De Paola says of the work,
“We’re very excited about the possibilities that the study opens up to learn about Down syndrome and the prospect that other neurodevelopmental and neurodegenerative disorders, such as schizophrenia, dementia or autism could also be modelled with our approach. I’m grateful to the many people who contributed to the study including the tissue donors and consider myself very lucky that Dr Raquel Real, a neurologist by training, chose to do her PhD with us and for her outstanding work in this area.”
The paper represents the PhD work of co-first author, Raquel Real, who discussed the study,
“We transplanted fluorescently labelled human neurons into the mouse cortex and then performed live imaging of both their structure and function over several months, as the cells matured. This has allowed us to explore the dynamics of neuronal development in a more physiological environment than an in vitro culture. This system has also proved powerful for modelling human neurodevelopment disorders, as we set out to investigate whether cells from individuals with Down syndrome showed developmental abnormalities. We found that these cells have a significant defect in their ability to form functional networks, which means neurons were less active and the communication between them less coordinated.”
Dr Real concludes:
“This finding could have a major impact on the development of cognitive symptoms in individuals with Down syndrome, and the next step is to understand why this occurs. If in the future we can pinpoint a specific mechanism, then an intervention could be possible.”
Raquel Real is about to complete her PhD in the Synaptic Plasticity and Repair group and has recently moved to UCL as a Clinical Research Associate in the Department of Clinical and Movement Neurosciences.