Research from the MRC Laboratory of Medical Sciences gives new meaning to phrases like “She gets that from her mother” or “Just like her dad”.
By LMS Staff Member
July 26, 2024
Time to read: 5 minutes
Females inherit two X chromosomes – one from each parent – along with all the other genetic material that builds and sustains the body. But despite this equal contribution from each parent, only one X chromosome is actively expressed in any given cell. Since the DNA sequence of each X chromosome is different, by expressing one X chromosome over the other a cell is effectively ‘choosing’ a set of unique characteristics to express.
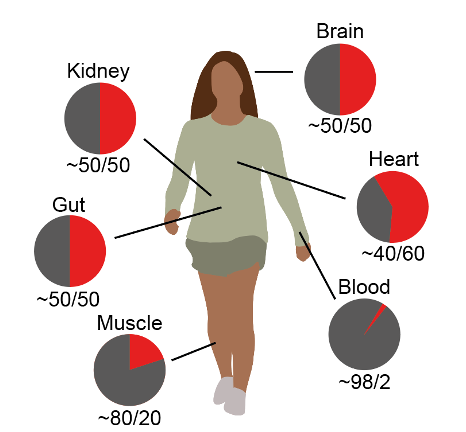
A new study from the Lymphocyte Development group and published in Nature Genetics reveals that the distribution of cells expressing the maternal or paternal X chromosome can be skewed in different parts of the body. The work also details a novel mechanism driving this skew: competition between rival sets of clones within each tissue of the body. As a result, in some areas the paternal X chromosome can dominate and in others it is edged out by the maternal X, meaning that your heart cells, for example, might be a little more ‘dad’, while your blood cells could have an extra hint of ‘mum’ – or even exclusively express one or the other X chromosome.We take for granted that we inherit half our genetic material from each parent, but new research from the MRC Laboratory of Medical Sciences (LMS) at Imperial College London casts that in a new light. The team looked at individuals who have two X chromosomes – females – and discovered that the contribution made by each chromosome can vary widely between cell types and tissues. That means DNA inherited from one parent is expressed more in some parts of the body, and the reverse in others – a finding that has fundamental implications for how female development takes shape, and could influence the likelihood of developing certain conditions later in life.
Each parent gifts us one full set of chromosomes, and the combined pairs contain all the genetic information to create the rich diversity of shape and function in the human body. One of these pairs determines biological sex, depending on whether an X or a Y chromosome is inherited from the father. One X is sufficient for many important biological functions, so in females (with two X chromosomes), one of the pair is silenced in every cell to ensure that the number of active X chromosomes is the equal between male and female cells. The choice of which chromosome each cell inactivates is random. This choice is then passed on by that cell to all its progeny through many rounds of cell divisions. This includes the founder cells to the many specialised cells and tissues that ultimately make up the human body.
In this way, two distinct lines of cells emerge which differ by which X chromosome is silenced and active. They are clones that carry identical genetic information but differ in which X chromosome they express (so identical genetics, but distinct epigenetics). No two human X chromosomes are exactly the same, due to variations in the genetic sequence on the maternal and paternal X chromosomes. Therefore, silencing one X chromosome means choosing to express one particular set of unique characteristics.
“We realised that when cells chose one of their two X chromosomes over the other, they also chose which set of genetic variants to express” said Matthias Merkenschlager, who leads the Lymphocyte Development Research Group. “As a result of X-linked variation and X chromosome inactivation, the two sets of clones found within each XX individual express distinct genetic variants.”
The researchers investigated the balance of these two cell clones throughout the body. They discovered that the choice of X chromosome dictates a cell’s odds of forming specific cell types. Those expressing one X-linked sequence variant were more likely to form certain cell types than others and vice versa.
But if the initial choice of X chromosome inactivation is random, why is there a skew in the final distribution of these clones? To better understand, the researchers focussed on a specific gene on the X chromosome, called Stag2. They found that cells with a variant of Stag2 were perfectly capable of forming immune cells called lymphocytes, but only if all cells expressed the variant gene (such as in males with just the one copy, or females whose two X chromosomes both carried the variant). However, if there were also cells present expressing the normal version of Stag2, rather than the variant, then the variant-expressing cells failed to produce immune cells. The mere presence of cells expressing the normal version was enough to prevent the variant cells from forming lymphocytes. This shows that epigenetically distinct clones expressing different X-linked variants compete for ‘permission’ to form specific cell types within the body. The findings reveal a new aspect of X-linked diversity not previously appreciated: that interactions between clones can shape the contribution of X-linked diversity to specific cell types and tissues.
Even if one clone outcompetes to form the blood, the other may predominate in other parts of the body. For the study’s lead author Teresa Buenaventura, this sparked a personal curiosity: “’Working on this project has been particular exciting for me since it has made me curious about the contribution of each of the X chromosomes to my different tissues,” she said.
Read the paper at Nature Genetics by clicking here.