 Lymphocyte Development group
Lymphocyte Development group
Moonlighting isn’t always easy to spot. After all, working at night doesn’t necessarily have to compromise the day job. Such is the case for cohesin, whose equivalent of a ‘day-job’ is to temporarily ‘glue’ duplicated DNA strands (chromatids) together before cells divide. A few years ago scientists at the CSC and elsewhere showed that the protein complex could also help switch genes on or off. Though unpicking this role from cohesin’s day-job in cell division proved a challenge.
However, now Vlad Seitan and colleagues from the CSC Lymphocyte Development Group – led by Matthias Merkenschlager and Amanda Fisher – have caught a glimpse of cohesin’s ‘moonlighting’ activities under circumstances in which the day-job – in cell division – is not required.
“T (immune) cells provide an ideal model to investigate cohesin’s other jobs, explains Vlad, “because as they mature, these cells have periods when they don’t divide.” Working in collaboration with scientists at Oxford and Duke Universities, he found a way to genetically modify mouse T cells to specifically knock out (turn off) cohesin at a point in the cell cycle where it isn’t needed for cell division.
And so cohesin’s new role in T cell differentiation – essentially, how they (T cells) take on certain characteristics to adapt to the range of biological invaders that they might meet – was discovered. “Cohesin is very important for the defining process in the development of T cells,” says Vlad. “It controls the recruitment of the highly specialised biological machinery that rearranges the Tcra locus”.
Tcra is part of the T-cell receptor (Tcr) complex, a ‘molecular aerial’ displayed on the surface of T-cells. Tcr can recognize and bind foreign particles, and the variable components of Tcra (that help them bind a specific invader) are generated using different combinations of sequences from Tcr genes. So-called rearrangement of Trca is integral to the ability of T cell populations to present a varied repertoire of aerials to invaders.
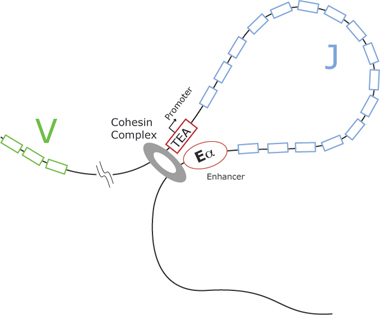
“We found that when we knocked out cohesin, there was a skewed repertoire of T cells,” Vlad confirms. “There were fewer types of T cell receptors, and the cells developed more slowly as a result.” Previous work in the group has shown that cohesin can influence the physical proximity of genes – the genomic architecture. “Now we show it can bring together the enhancer and promoters (elements of DNA that allow genes to be switched on and off) of the Tcra locus.
Although it’s doing a different job, the way cohesion works is similar ‘by night and by day’. The ring-shaped protein complex behaves like a hairband stabilising a plait when it brings chromatids together before a cell divides. To bring promoter and enhancer together, Vlad and co-workers envisage a similar model to facilitate DNA looping.
“Cohesin directly controls transcription,” says Vlad. “It affects how much, but also where DNA gets transcribed. Without it the architecture of the Tcra locus is disrupted and transcription hampered. Transcription at the Tcra locus not only boosts production of Tcra proteins,” he adds, “it also acts as a beacon for the rearrangement machinery.”
“The protein complexes that generate Tcra variability (by shuffling DNA sequences) only bind to sites of active transcription.” DNA not being transcribed is ignored. “Transcription failure in T cells without cohesin led to many variable segments being ignored during rearrangement,” he clarifies. “So fewer types of T cell receptor can form.” Further investigation will no doubt clarify the full moonlighting capacity of cohesin, and the implications for immunity and development.
(Illustrative impression)
Tcra locus diversity, which lends the immune system some of its adaptability, depends upon the combination of about 60 ‘J’ (joining) exons with 100 ‘V’ (Variable) exons. Cohesin plays a major role in the combination mechanism and allows the formation of a vast array of T cells with different receptor forms. When cohesin is missing, this combination mechanism is much less efficient and there is a skewed or limited repertoire of receptor types, with implications for a reduced immune response.
BM/SJ
Seitan et al. (2011) A role for cohesin in T cell receptor arrangement and thymocyte differentiation. Nature, in press. dx.doi.org/10.1038/nature10312
