By Sophie Arthur
April 1, 2019
Time to read: 4 minutes
People are living longer than ever before globally. While this is a major achievement of modern medicine and healthcare, it does mean that a growing proportion of the population is made up of older people and all the associated implications. A report in 2018 from the Office of National Statistics projects that by 2066, a quarter of the UK population will be aged 65 or over. This highlights, more than ever before, that we need to find ways to help our aging population age healthily. Almost all aging individuals show bone loss leading to increase in risks of fracture, which also take longer to heal than a younger adult, and are more likely to suffer from conditions like osteoporosis and arthritis.
Research published on 1 April in Nature Cell Biology by the Integrative Skeletal Physiology group at the LMS has uncovered how the blood vessels in bone are involved in regulating bone formation, which could have implications for a healthy aging population.

Whether it is during normal growth and development or during healing or repair, most types of bones are formed through a process involving, first, the formation of an intermediate cartilage structure. For example, the elongation of long bones happens through the conversion of growth plate cartilage, which is found at either end of the bone, into bone tissue. The cartilage structure is broken down by cells called osteoclasts, before another type of cell called osteoblasts help lay down the bone tissue around the vast network of blood vessels that can be found there. Though bones are highly laced with capillaries, we are at the early stages of understanding the functions of these blood vessels.
In this study, researchers showed that a specialised subtype of capillaries, termed type H, play a central role in replacing the cartilage matrix with bone. Specifically, the endothelial cells that associated with the capillaries had this proteolytic activity, or ability to breakdown and digest the cartilage matrix, that was earlier thought to belong to osteoclasts. This is also a previously unknown function of these blood vessel that is reported in this study. Moreover, these capillaries were found to control the growth direction of bone, as bone growth was always orientated towards the blood vessels. When the researchers changed the direction at which the blood vessels were growing, the direction of bone growth also changed. This highlighted how these endothelial cells from blood vessels can shape bone elongation by digesting the bone matrix.
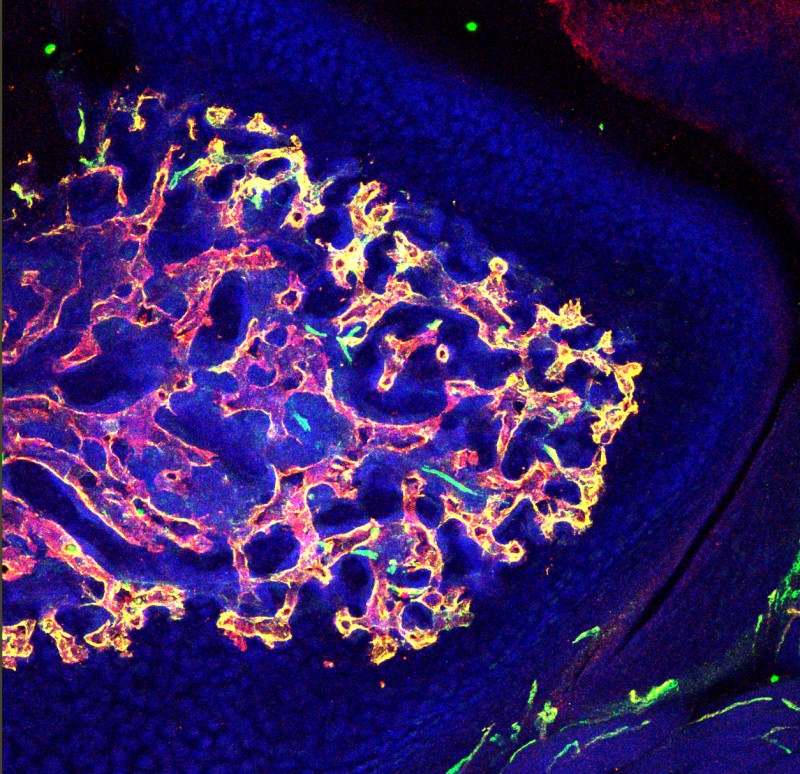
This transverse section of bone shows distribution of blood vessels near cartilage region. Blood vessels were immunostained for the expression of markers, CD31 (green) and endomucin (red). Type H capillaries (yellow) are in close proximity to chondrocytes. DAPI (blue) is a nuclear counterstain.
But these endothelial cells also have a support act. This study also identified a new type of osteoclast. This cell type isn’t able to digest the bone matrix, but instead helps the blood vessels in their directional growth.
How could this research help our aging population?
As previously mentioned, an aging population means more strain on healthcare services with regards to fracture healing and conditions like arthritis. In fractures, for example, cartilage models need to be laid down before they are removed to make way for new bone. But this repair process takes much longer in older people, so this research could be used to accelerate the function of the endothelial cells to improve the repair more quickly. On the other hand, in conditions like arthritis, there is excessive cartilage damage and excessive blood vessel infiltration, so this research could help pave ways to stop the action of these endothelial cells in arthritis to reduce cartilage damage. This is exactly the direction that these researchers want to take this research next.
Saravana Ramasamy, Head of the Integrative Skeletal Physiology Group, discusses the next steps:

“We have uncovered this interesting function of blood vessels, but the mechanism of how we can control this function is not very well understood. However, it is clear that blood vessels are involved in shaping the architecture of bone tissue. Therefore, we want to move forward by collaborating with fracture repair and arthritis animal models to investigate whether we can locally control the proteolytic actions of the endothelial cells”
‘Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation’ was published in Nature Cell Biology on 1 April.