Our lab is interested in the transcriptional and epigenetic regulation of early development. We study how transposons have been co-opted to regulate essential processes in development, and how their misregulation may contribute to disease.
“Why do some essential parts of our DNA cause diseases later in life?”
In the early stages of foetal development, cells have the potential to develop into any adult cell type. This ability is called pluripotency, and it is regulated by a complex array of genetic mechanisms.
Our research focuses on portions of our genomes called Transposable Elements (TEs). These are mobile DNA elements that can make copies of themselves and integrate into new positions within the genome and they work together to influence cell development. However, their reactivation later on in life has also been linked to disease development.
We are using mouse and human embryonic stell cells as well as mouse embryo models to find out more about the effects of TEs and how they influence early foetus development.
Our group is using CRISPR technology, imaging and bioinformatics to study these TEs at a genome-wide level as well as focussing on particular TEs of interest.
Development of several diseases, including some types of cancer, have been linked to reactivation of these TEs.
We want to understand more about how these understudied portions of our genome are regulated and the detrimental effects that occur if they are not regulated properly.
“We study how transposons have been co-opted to regulate essential processes in development, and how their misregulation may contribute to disease.”
In early development, a single fertilized zygote proceeds through a series of cleavage steps to develop into a multicellular blastocyst. The cells of the blastocyst are capable of generating all adult cell types, a phenomenon known as pluripotency. The inner cell mass (ICM) of the blastocyst can moreover be cultured in vitro as pluripotent embryonic stem (ES) cells, which have become invaluable tools for understanding development and for regenerative medicine.
The broad focus of our lab is the transcriptional and epigenetic regulation of early development. In particular, we are interested in understanding the importance of transposable elements (TEs) in development and disease. TE sequences make up nearly 50% of our genomes, yet have been greatly understudied. TE reactivation has been recently linked to several diseases such as cancer and neuroinflammation. Intriguingly however, our work as well as several others’ has highlighted the dynamic expression of several TE families during normal development, as well as their functional importance in embryogenesis.
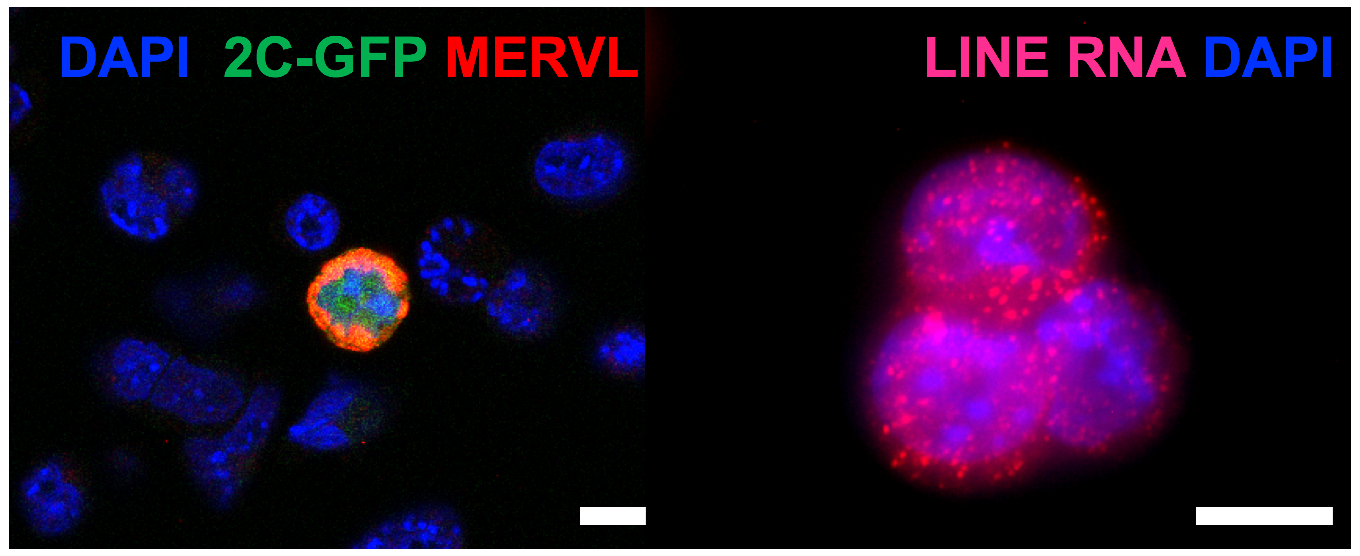
Using mouse and human ES cells and mouse embryo models, we are investigating how TE networks function in distinct stages of mammalian development. We employ a combination of candidate and genome-wide approaches, CRISPR technology, imaging and bioinformatics to describe and dissect TE function in embryogenesis and cell fate choices. We additionally explore how pathways that regulate TE expression may fail in cases of disease.
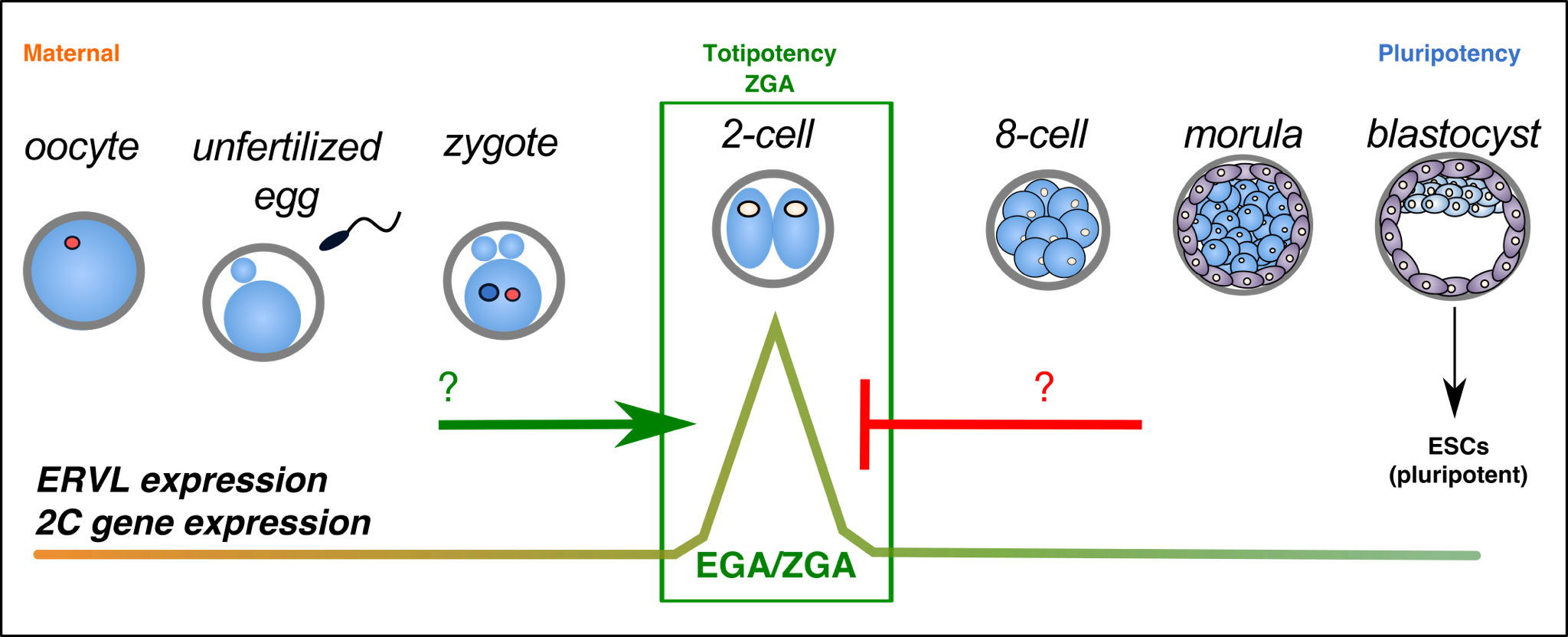
Mechanisticly, the MERVL activator, Dux, becomes recruited to perinucleolar heterochromatin for its repression at the 2-cell stage, and disruption of nucleolar integrity and phase separation is sufficient to activate totipotency features.
These results reveal a novel link between the nucleolus, gene and TE regulation, and cell fate. We are now exploring the role of the nucleolus in human development, the genome-wide roles of nucleolar chromatin, and the link between nucleolar dysregulation and disease.
Michelle Percharde holds a UKRI Future Leaders Fellowship


Reen, V., D’Ambrosio, M., Bogaard, P. P., Tyson, K., Leeke, B. J., Clement, I., Dye, I. C. A., Pombo, J., Kuba, A., Lan, Y., Burr, J., Bomann, I. C., Kalyva, M., Birch, J.. Khadayate, S., Young, G., Provencher, D., Mes-Masson, A., Vernia, S., McGranahan, N., Brady, H. J. M., Rodier, F., Nativio, R., Percharde, M., McNeish, I. A. & Gil, J. (2025). SMARCA4 regulates the NK-mediated killing of senescent cells. Science Advances. DOI: 10.1126/sciadv.adn2811
Chammas, P., Xie, S. Q., Sepulveda-Rincon, L. P., Leeke, B J., Dore, M. H., Dormann, D., Wagner, R. T., McManus, M. T., Karimi, M. M., Young, G. & Percharde, M. (2024). CRISPRa-mediated disentanglement of the Dux-MERVL axis in the 2C-like state, totipotency and cell death. bioRxiv. https://doi.org/10.1101/2024.11.25.625195;
De Souza, R. A., Barneda, D., Karimlou, D., Bovee, N. G., Zheng, Y., Kahlman, E. J., Novo, C. L., Ellis, J. K., Leeke, B. J., Bangalore, M. P., Liu, Z., Sousa, B. C., Lopez-Clavijo, A. F., Jansen, J. H., Barahona, M., Percharde, M., Keun, H. C., Christian, M., Marks, H., & Azuara, V. (2024). The exit of naïve pluripotency contains a lipid metabolism-induced checkpoint for genome integrity. bioRxiv (Cold Spring Harbor Laboratory). https://doi.org/10.1101/2024.09.22.613751
Mau, K.H.T., Karimlou, D., Barneda, D., Brochard V., Royer C., Leeke B., A de Souza R., Pailles M., Percharde M., Srinivas S., Jouneau A., Christian M., Azuara V. (2022) Dynamic enlargement and mobilization of lipid droplets in pluripotent cells coordinate morphogenesis during mouse peri-implantation development. Nat Commun 13, 3861. https://doi.org/10.1038/s41467-022-31323-2
Xie SQ, Leeke BJ, Whilding C, Wagner RT, Garcia-Llagostera F, Low XY, Chammas P, Cheung NT-F, Dormann D, McManus MT, Percharde M. (2022) Nucleolar-based Dux repression is essential for embryonic two-cell stage exit. Genes Dev, Mar 10. doi: 10.1101/gad.349172.121
Percharde M, Sultana T & Ramalho-Santos M. (2020). What Doesn’t Kill You Makes You Stronger: Transposons as Dual Players in Chromatin Regulation and Genomic Variation. Bioessays; 42, 4.
Lu JY, Shao W, Chang L, Yin Y, Li T, Zhang H, Hong Y, Percharde M, Guo L, Wu Z, Liu L, Liu W, Yan P, Ramalho-Santos M, Sun Y, Shen X. (2020). Genomic Repeats Categorize Genes With Distinct Functions for Orchestrated Regulation. Cell Reports,10;30(10):3296-3311.e5
DiTroia SP, Percharde M, Guerquin M, Wall E, Collignon E, Ebata KT, Mesh K, Mahesula S, Agathocleous M, Laird DJ, Livera G, Ramalho-Santos M. (2019). Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature. 573, 271-275.
Percharde M, Lin C-J, Yin Y, (2018). A LINE1-Nucleolin Partnership Regulates Early Development and ESC Identity. Cell. 174, 391-405.
Bulut-Karslioglu A, Macrae TA, Oses-Prieto JA, (2018). The Transcriptionally Permissive Chromatin State of Embryonic Stem Cells Is Acutely Tuned to Translational Output. Cell Stem Cell. 22, 369-383
Percharde M, Wong P, Ramalho-Santos M. (2017). Global Hypertranscription in the Mouse Embryonic Germline. Cell Reports. 19, 1987-1996.
Percharde M, Bulut-Karslioglu A, Ramalho-Santos M. (2017). Hypertranscription in Development, Stem Cells, and Regeneration. Developmental Cell. 40, 9-21.
Qin H, Hejna M, Liu Y, (2016). YAP Induces Human Naive Pluripotency. Cell Reports. 14, 2301-2312.
Percharde M, Lavial F, Ng J-H, (2012). Ncoa3 functions as an essential Esrrb coactivator to sustain embryonic stem cell self-renewal and reprogramming. Genes & Development. 26, 2286-2298.