A crucial step in DNA replication that could prevent mutations and disease has been described by researchers from the MRC Laboratory of Medical Sciences (LMS). The research sheds light on how a quality control mechanism prevents DNA replication if the molecular machinery for copying DNA is incorrectly assembled.
By Tom Wells
January 6, 2025
Time to read: 4 minutes
Each time a cell divides, it must first copy its DNA. Any errors that occur during DNA replication will be passed on to the daughter cells. With each division the copying mistakes accumulate. These errors cause genetic changes that can lead to illnesses such as cancer. As such, DNA replication must be accurate and tightly controlled.
One way a cell ensures DNA replication is accurate is through a pre-replicative complex (pre-RC). The pre-RC is a large protein complex that binds to DNA and then recruits the proteins responsible for DNA replication.
If the pre-RC is incorrectly assembled because one of the proteins in the complex is mutated or damaged, the system will fall apart without replicating DNA. This is an important DNA quality control mechanism, which helps to prevent against mutations that can cause disease such as cancer.
In research published last week in Nature Communications, work led by Dr Sarah Faull and Dr Marta Barbon in the DNA replication group at the LMS, headed by Professor Christian Speck, sheds light on how the pre-RC in yeast falls apart if incorrectly assembled. Researchers already knew that the pre-RC could check its structure and then either disassemble if the structure isn’t correct or recruit the proteins required for DNA replication.
The pre-RC is made up of many components and many do not have clear functions. One region of the pre-RC that is not well understood is the end of a subunit of the pre-RC called Mcm5. The end of this subunit, often called C5, previously could not be visualised in the pre-RC complex. To find out more about this subunit, the team investigated yeast that lacked C5. These yeast had much slower growth, suggesting C5 plays a key role. Using an advanced microscopy technique called cryo-EM, it was possible to see that C5 acts as a plaster holding the complex together.
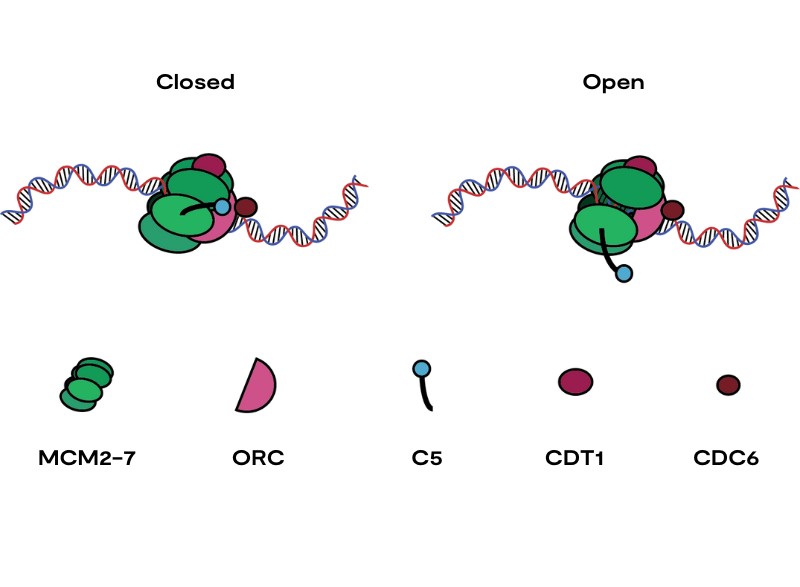
To check its structure, the pre-RC needs energy. This is found in a molecule called adenosine triphosphate (ATP). ATP is often called the energy currency of biology. This is because biological processes can be powered by the energy released when one of its three phosphate molecules is removed and it becomes adenosine diphosphate (ADP), in a process called ATP hydrolysis.
Before this paper, scientists thought that part of the pre-RC called Cdc6 was responsible for ATP hydrolysis to generate the energy needed for quality control. However, this novel work has identified that a different part of the pre-RC is responsible. By testing pre-RCs that contained modified proteins, they found that Mcm4 is hydrolysing ATP in the structural check point.
“This discovery is exciting and unexpected – when a PhD student in our lab, Marta Barbon, started working on C5 a few years ago we had no idea it would lead to uncovering the key protein involved in ATP hydrolysis in pre-replication complex formation,” says Sarah.
The team also saw that disassembly occurred by the Mcm complex splitting in half. This was surprising as before scientists thought that the pre-RC just fell apart by DNA exiting the Mcm gate, in the same way that it enters the complex.
“We’ve discovered that the motor protein Mcm4 promotes successful pre-replicative complex formation and the disassembly of non-productive complexes,” concludes Christian, “This explains how DNA replication is controlled to support genome stability and organism longevity.”
While this work was done in yeast, it sets the groundwork for better understanding of how our own cells copy their DNA, potentially leading to future therapies by understanding the quality control mechanisms that underly DNA replication.
This work was funded by the BBSRC, the MRC, the Wellcome Trust and Cancer Research UK.
Faull, S.V., Barbon, M., Mossler, A. et al. MCM2-7 ring closure involves the Mcm5 C-terminus and triggers Mcm4 ATP hydrolysis. Nat Commun 16, 14 (2025). https://doi.org/10.1038/s41467-024-55479-1
Published 2 January 2025
Sarah has also described the work in a blog for “Behind the Paper”, a series of community blogs from authors of Nature research publications.