We study how the different layers of information encoded in our genomes, the basic building block of life, impact whether we get diseases such as obesity and diabetes. We focus on fat tissues which have crucial functions beyond fuel storage that alter health and lifespan.
Fat tissues store and release fuel to meet energy needs, maintain normal blood sugars, regulate body temperature, and make hormones that control appetite. We study how the genome coordinates cells in fat tissues to fulfil these functions, how having too much fat (obesity) hijacks this, and how this leads to health problems like diabetes and heart disease. We do this in people from diverse backgrounds across the life course, so we can improve treatment options, patient outcomes and health equity.
One area we work on is epigenetic switches on DNA that turn genes on and off without altering the genetic code. These switches are implicated in many aspects of obesity and diabetes. But finding and proving which switches cause human disease is a big challenge. We use innovative screening and refinement strategies to identify switches in human fat that may contribute to obesity and diabetes, and genes and proteins these switches are likely to affect.
Another area we study is how weight gain causes diabetes and how weight loss can put diabetes into remission. One approach we use is single cell sequencing. This allows us to collect detailed information about gene activity in every cell in a tissue using a single test. By profiling thousands of cells in fat donated by people with different body weights, we seek to pinpoint cells and genes that may trigger, exacerbate, or reverse diabetes.
We then evaluate in the lab what these switches, genes and proteins do and whether they might be modified to treat the harmful health consequences of obesity.
Our research focuses on understanding the impact of genetic and environmental risk factors, and therapeutic interventions, on human obesity and diabetes.
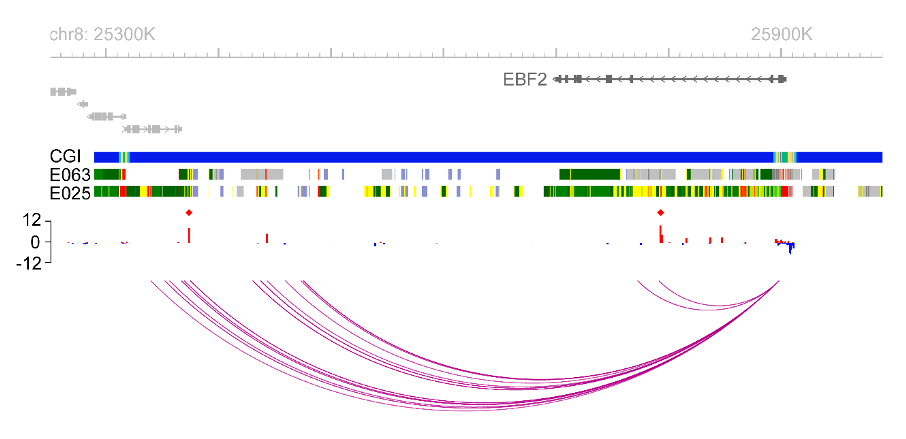
We use high-throughput molecular screens in deeply phenotyped human cohorts to identify genetic, epigenetic, transcriptomic and proteomic variations linked to disease, and advanced experimental tools to assign causation and mechanism to these changes. With this strategy, we have recently discovered epigenetic modifications to the genome in human fat cells, and refined these to sites that may have causative roles in obesity, insulin resistance and diabetes. Illustrated (below) at the EBF2 locus which controls thermogenic energy expenditure. We have also systematically characterised cell-type specific gene regulatory networks and molecular signalling pathways in human fat tissues, that may underlie the beneficial effects of weight loss on metabolic health. We are currently investigating how our top leads contribute to disease processes, and whether these might be modified for therapy.
Ultimately, we aim to advance our findings into better treatments for obesity and diabetes.
ORCID: https://orcid.org/0000-0001-5467-114X
We study how adipose tissues remodel in human obesity and weight loss, and how this leads to the development and remission of diabetes, by integrating findings from human tissues and experimental models.
This approach has enabled us to define critical tissue injury and repair pathways linked to clinical outcomes. Building on our discoveries, we are using cell screens to identify proteins and compounds that modify these pathways; and ii. machine learning to simulate how pathways, cells, tissues, and individual patients will respond to targeted therapeutic interventions.
Ultimately, we aim to accelerate the development of pharmacotherapies that target adipose tissues to treat diabetes and other adverse complications of obesity.
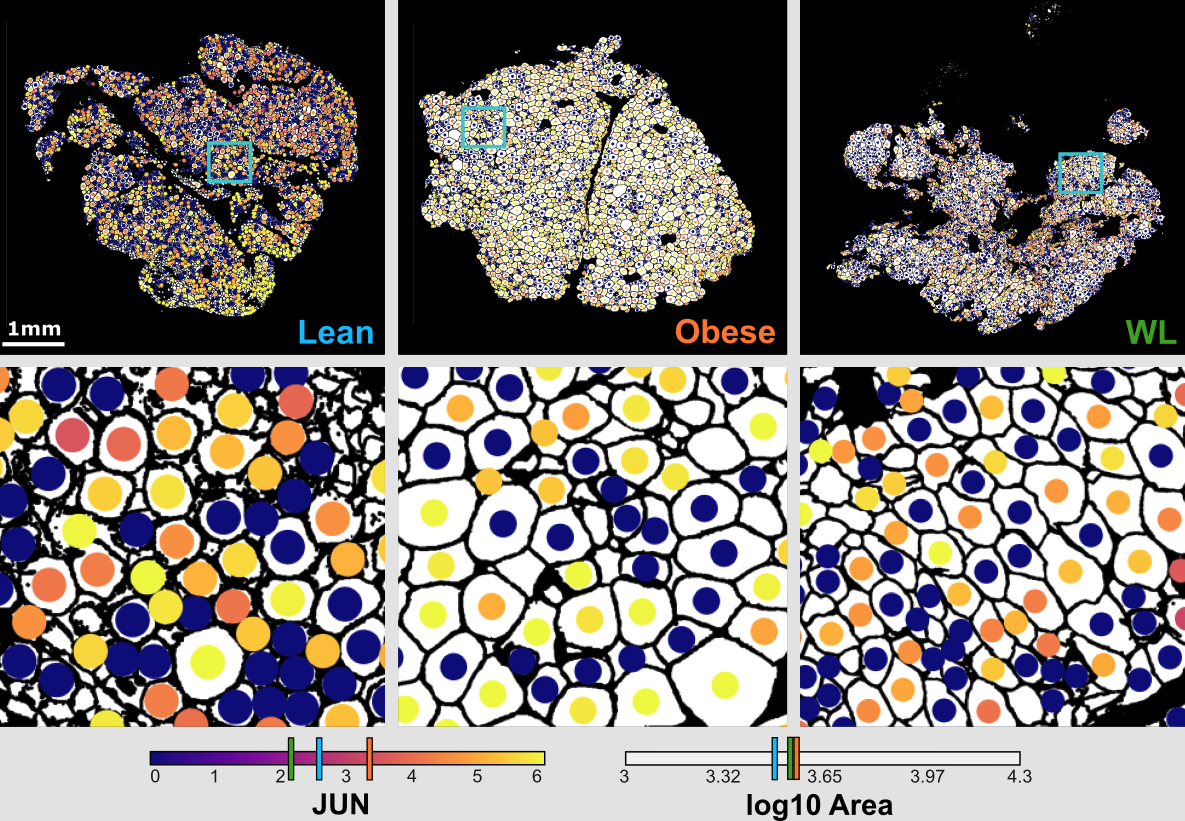
Adipocyte (fat) cell stress, exemplified here by expression of the JUN gene, in association with change in cell size (hypertrophy) in human obesity and weight loss.





A Miranda et al. Selective remodelling of the adipose niche in obesity and weight loss. Nature. 2025. 644, 769–779. *Corresponding author.
L McAllan et al. Integrative genomic analyses in adipocytes implicate DNA methylation in human obesity and diabetes. Nature Communications. 2023. 14, 2784. *Corresponding author.
J Hawe et al. Genetic variation influencing DNA methylation provides insights into molecular mechanisms regulating genomic function. Nature Genetics. 2022 Jan;54(1):18-29.
S Wahl* … WR Scott* et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017 Jan 5;541(7635):81-86. *Joint lead author.
WR Scott et al. Investigation of Genetic Variation Underlying Central Obesity amongst South Asians. PLoS One. 2016 May 19;11(5):e0155478
B Lehne et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 2015 Feb 15;16:37.