By Staff Member
November 27, 2023
Time to read: 6 minutes
Scientists from the MRC-LMS (Laboratory of Medical Sciences) and their collaborators have uncovered critical insights that can pave the way for novel therapeutic approaches to tackle cancer, fibrosis, and many age-related conditions. Their research has identified a new pathway serving as a target for a class of drugs which selectively eliminate cells which are known to provoke inflammation. The drugs, known as senolytics, eliminate cells which have entered a state of senescence, ‘zombie cells’, effectively; and in doing so can prevent tissue damage and bolster immunity. Already commercial partner Myricx Bio is applying these discoveries to advance highly targeted innovative cancer drugs called ADCs.
Senescent cells are cells taking a break – they have stopped multiplying, usually in response to cell damage or ageing. These cells, however, continue to release proteins that can induce inflammation in the body. Normally the body eliminates these cells through our immune system, however ageing and disease can disrupt this process. As a result, senescent cells can accumulate in the body, causing chronic inflammation and tissue disruption. This further fuels the ageing process and disease development, which in turn generates more senescent cells, forming a vicious cycle.
By removing senescent cells, senolytics drugs reestablish tissue homeostasis improving the outcome of many age-related diseases. Though promising in pre-clinical studies, there are currently no senolytics on the market, and those in clinical trials have limitations, calling for the exploration of more effective options.
“Our team has long been looking into senolytic targets that effectively and selectively kill senescent cells, “says Prof. Jesus Gil, lead author of the study and head of the Senescence Research Group at LMS. “In a previous study, we showed the potential of repurposing existing drugs, but there are only limited drugs to choose from. In this study, we expanded our selection pool much larger by seeking targets in over 7,000 ‘druggable’ genes. We were thrilled to reveal previously unknown vulnerabilities of senescent cells. This opens up new possibilities for treating age-related diseases.”
The team delved into the molecular pathways influencing the survival of senescent cells. By using an approach called RNA interference (RNAi), which prevents protein production by reducing gene expression, they looked for genes that are essential for the survival of senescent cells. They screened RNAi molecules targeting over 7,000 genes and selected the RNAi molecules that selectively killed senescent cells, but not normal cells. This screen identified a critical senolytic target pathway called coat protein complex I (COPI). Inhibiting this pathway, which is responsible for carrying proteins in the cells, triggered a cascade of events that led to the death of senescent cells. Furthermore, they also showed that targeting this pathway improved outcomes in mouse models of cancer and fibrosis.
Building on the COPI breakthrough, the researchers explored drug candidates that target the COPI pathway. Although there are some existing drugs that directly interfere with the COPI pathway they have limited effectiveness because they only have a short lifespan in the bloodstream so were not considered suitable for clinical use. ARF1, a key regulator of COPI vesicle formation needs a lipid modification (called myristoylation) to be functional. The LMS team collaborated with Prof Edward Tate at Imperial College London and Myricx Bio, an Imperial spin-off, to investigate whether a different class of drugs which indirectly inhibit the COPI pathway, N-myristoyltransferase inhibitors (NMTi), could also be senolytic. The results were positive- NMTi demonstrated potent senolytic effects, effectively improving the outcome of cancer and fibrosis in mouse models.
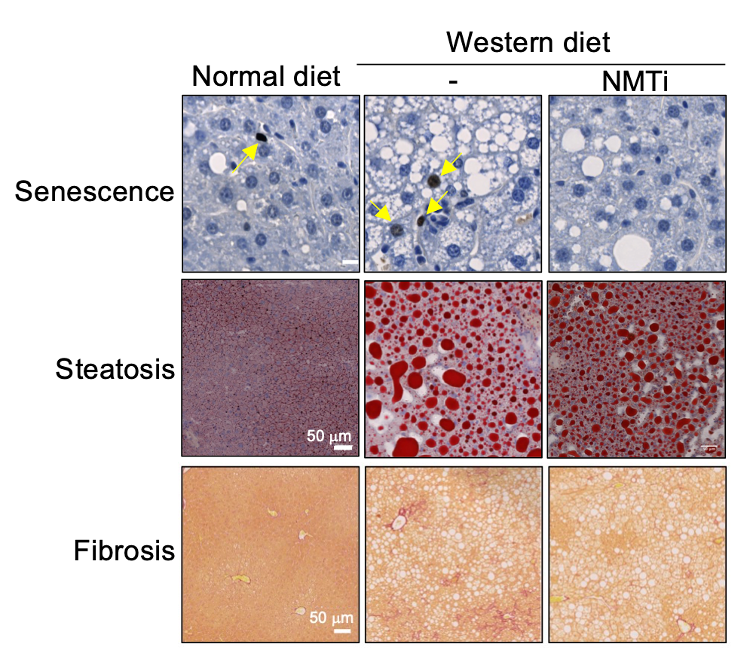
Liver sections showing how the treatment with NMTi reduced cellular senescence, the accumulation of fat (steatosis) and fibrosis in mice fed with a Western diet (a model for non-alcoholic steatohepatitis)
“A big part of the success of the project was thanks to the close collaboration with Ed Tate’s lab and all of the Myricx team, if I have to single out someone Roberto Solari, a Myricx Bio co-founder was always there to give advice and see how we could push the project forward” commented Jesus Gil.
“Our findings suggest that NMT inhibition could be a game-changer in senolytic therapy. Our potent, selective, in vivo active small molecule NMT inhibitors open exciting avenues for further research and development, as the first senolytics targeting protein lipidation.” commented Prof Edward Tate, GSK Chair in Chemical Biology at Imperial College London, and Satellite Group Leader at the Francis Crick Institute.
CEO of Myricx Bio, Dr Robin Carr, an experienced pharma drug hunter said “We are combining highly potent inhibitors of NMT with antibodies that are targeted to specific cancer cells to create novel biologic drugs called antibody drug candidates. The senolytic properties that this research has demonstrated give our NMTi-ADCs the potential for a highly differentiated profile not addressed by any other ADCs. Combined with the cytotoxic effect of NMTi, the senolytic mechanism has the potential to induce deeper and more durable responses than purely cytotoxic payloads. We look forward to exploring this as we advance our drugs into human clinical trials.”
Lead author Prof Jesus Gil highlighted, “This work defines a novel class of senolytic that expand the possibilities to treat a wide range of diseases associated with senescence including cancer, idiopathic pulmonary fibrosis (IPF) or non-alcoholic steatohepatitis (NASH).”
The study opens up a whole new avenue of opportunity to develop drugs which can attack major diseases such as cancer, fibrosis, and other ageing-related conditions. The team is now looking ahead to further research and potential clinical trials, representing a promising advancement in the quest for healthier aging.
The study was published in Nature Cell Biology Nov 27th, 2023, as a collaborative effort of research groups at LMS, Imperial College London, University College London, The Institute for Research in Biomedicine from Barcelona, University of Southampton and The Francis Crick Institute. Myricx Bio, an Imperial spin-off, provided access to the NMTi used in the study. UKRI, through MRC core funding and CRUK were the main funders of this study, a full list of funders is included in the paper.
Read the paper here.