By Sophie Arthur
January 29, 2021
Time to read: 5 minutes
By Dr Sophie Arthur
When you imagine a brain cell, you imagine the ‘star-shaped’ body of the cell and the long and slender projection called an axon. The axon acts like a wire or a cable in your brain and transmits the electrical signals to different brain cells, or neurons, or to other parts of our body. It will perhaps then come as no surprise that these axonal connections are vulnerable to injury, and when they are lost, that communication between the brain and the rest of the body also disappears. This often has irreparable consequences as many brain and body functions (such as retrieving a memory or moving) depend on those axonal connections. Precious connections are lost upon axon degeneration, a hallmark of many neurological conditions such as Alzheimer’s, multiple sclerosis, stroke and Parkinson’s disease. So, understanding the mechanisms of how axons degenerate is going to be crucial to be able to prevent or delay it happening, and it also opens up a new avenue of potential therapeutics to treat brain disorders.
Wallerian degeneration (named after Augustus Waller, the British scientist who first described it in the late 19th century) is a tightly regulated form of axon degeneration after injury. It has been well studied in spinal cord and optic nerve injury models, but to a lesser extent in the more complex nature of the brain. It is an active process devoted to the removal of disconnected axons and is conserved across different species. Once disconnected, axons never reconnect in mammals, and always eventually degenerate. One major feature of this type of degeneration though is that it is very slow. It could take as long as a few days from start to finish. But why this process is so slow has remained unsolved.
Axon degeneration occurs frequently throughout the injured and diseased nervous system, but the key steps of how this happens are not fully understood, particularly in the brain where neuronal networks are very complex. However, research from the Synaptic Plasticity and Repair group at the MRC LMS, published in the journal BMC Biology, uses a powerful live imaging technique to capture what happens when axons become transected, and in a technical tour de force they observe for the first time this process from start to end in the mammalian brain.
Using a non-invasive microsurgery method, the researchers use a laser to make a precise cut to an axon in the otherwise intact mouse brain, mimicking damage to kickstart the degeneration process. Using fluorescence to observe just a few individual axons among millions of others, the team finds that if a disconnected axon is below a certain length, it goes through an uncharacteristically quick Wallerian degeneration – ‘so-called’ rapid onset Wallerian degeneration. These axons are degraded over a few hours – which is substantially quicker than the usual few days reported for this type of degeneration. In fact, a fragment of the axon with a defined length degenerates with similar speeds no matter where it is along the length of the axon.
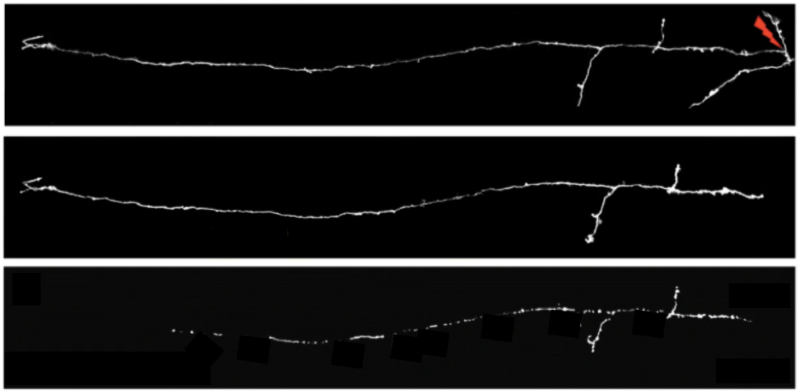
This suggests that there is either a protective factor that is lost, or a trigger that would need to accumulate in order for degeneration to occur, which then results in the rapid disappearance of the disconnected axon segments. They next investigate whether the density of synaptic connections or axon complexity matter. They find that Wallerian degeneration is dependent on synaptic density, i.e. that the more connections, the slower the degeneration, but it is independent of the axon complexity.
Finally, they use pharmacological and genetic manipulations to show that a mechanism based on nicotinamide adenine dinucleotide (NAD), a derivative of vitamin B, can delay the rapid onset Wallerian degeneration in these short axon segments, demonstrating conservation of the molecular mechanisms controlling Wallerian degeneration in different areas of the mammalian nervous system.
It is still unclear why this fast degeneration occurs in the brain, the authors speculate it could have evolved to rapidly clear unwanted debris from the brain, but this study shows that we need to investigate axonal degeneration in the mammalian brain further, especially if we want to find more effective ways to treat brain disorders where axon degeneration is known to occur.
Vincenzo De Paola, Head of the Synaptic Plasticity and Repair group of Imperial College London and Honorary Group Leader at the MRC London Institute of Medical Sciences, the senior author of this paper, shared this about this project and the next steps:
“This project was an imaging ultra-marathon. I must praise the heroic effort of my team, especially Associate Professor Alison Canty and Dr Johanna Jackson, the two brilliant post-docs who spearheaded this project with help of many other talented researchers, as they worked in shifts and often through the night in order to capture all the time points required to determine when the degeneration was happening and at what speed. It really was an impressive team effort to pull together the data set, ‘a labour of love’ as they described it themselves.
As for next steps, a recent joint study by the Vanderhaeghen and De Strooper labs (VIB, Belgium and University College London, UK, respectively) pointed to different vulnerability of human neurons to the toxic factors in Alzheimer’s disease, compared to mouse neurons. So, we would like to look and see how the findings of rapid onset Wallerian degeneration uncovered in mice apply to human neurons too. We recently developed a system where we can use the same powerful microscopes to follow individual human axons growing inside a small piece of brain tissue created from stem cells. It will be fantastic to study axon and synaptic degeneration and possible repair strategies for the first time in a human experimental model.”
‘In vivo imaging of injured cortical axons reveals a rapid onset form of Wallerian degeneration’ was published on 18 November in the journal BMC Biology. Read the full article here.