Being too clean might be bad for your descendants, according to new research from scientists at the LMS.
In research published in the journal BMC Biology, the Transgenerational Epigenetic Inheritance and Evolution group found that the offspring of nematode worms infected with pathogenic bacteria grew significantly better than the offspring of uninfected worms. Their findings provide important new insight into the elusive function of transgenerational epigenetic changes, and also have interesting implications for human health.
Some epigenetic changes can be passed on from parent to offspring
Every cell in the human body has the same DNA sequence, yet different cells have distinct functions. This is because different cell types use different sets of genes within the human genome, turning some genes off permanently and activating others. When the cell divides, the information about which genes are switched off and which are switched on is transmitted so that the cell “remembers” what it is supposed to be doing. This type of information is known as “epigenetic” because it is heritable through cell division, yet does not involve any change in the sequence of the DNA itself.
It’s best understood as how an organism develops from a single cell. However, recently, several examples have been found where particular epigenetic changes can be passed on between generations in a process known as transgenerational epigenetic inheritance. So far how these examples, found in the laboratory relate to the life of organisms in the wild is still a mystery.
Exploring the role of epigenetics in adaption to the environment
The Transgenerational Epigenetic Inheritance and Evolution group set out to try to understand how transgenerational epigenetic changes could contribute to the adaptation of organisms to their environment. They decided to study the process in a type of nematode worm, called Caenorhabditis elegans (C.elegans). Transgenerational processes are easy to study in these worms as they have a very short generation time, 4 days, compared to 22-33 years in humans. In these worms, it is known that transgenerational effects are established by molecules called PIWI-interacting RNA (piRNAs), tiny RNA molecules that can silence particular genes. The group reasoned that if the piRNA pathway responded to different stimuli from the environment, this might provide clues as to what role transgenerational epigenetic inheritance might have in adapting to different conditions.
“We designed a screen, using an artificial gene called Green Fluorescent Protein (GFP) that we engineered to be switched off by piRNAs. We could use this as a sensor to see how many piRNAs were being produced in the worms: if the worms turned green then we knew that the piRNA pathway was less active,” said Tony Belicard, the postdoctoral researcher who carried out the screen.
The scientists found that fewer piRNAs were produced when the temperature was raised to 25°C from the normal temperature of 20°C, and the worms turned green. However, they also found a surprising impact when the worms were infected with pathogenic bacteria such as the human pathogen Pseudomonas aeruginosa. The pathogenic bacteria reversed the effect of temperature such that more piRNAs were produced and the green colour went away (see image below). The team showed that there were differences in gene expression in the offspring due to transgenerational epigenetic changes established by piRNAs. These changes correlated to changes in the fitness of the offspring of the infected worms. The offspring of infected worms grew much better at 20°C than the offspring of uninfected worms.
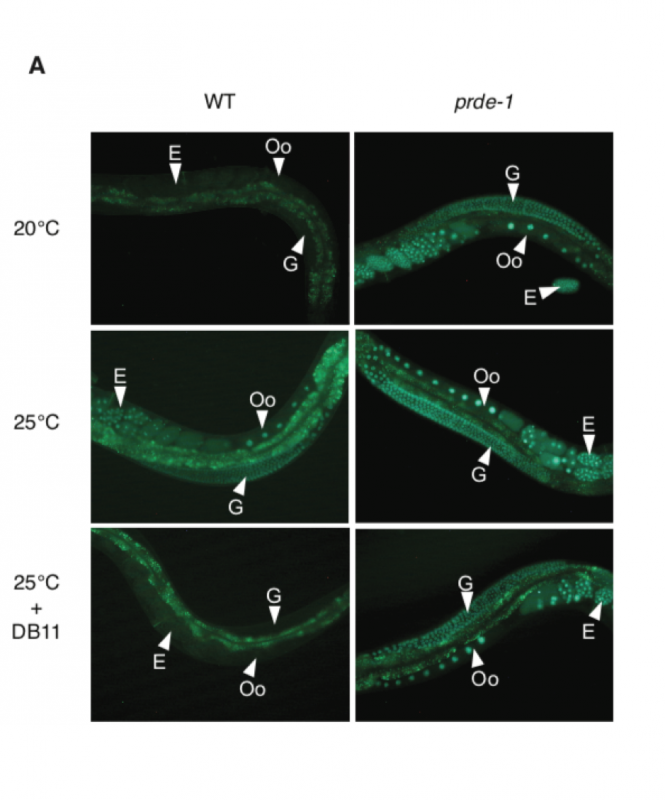
Images of worms grown at different temperatures, with (WT) or without the piRNA pathway removed (prde-1). The top row of worms grown at 20°C silence the piRNA sensor (left) unless the piRNA pathway is removed (right). The middle row at 25°C, the piRNA pathway is reduced and the piRNA sensor becomes visible, illustrated by the increased green fluorescence. In the bottom row, when infected with pathogenic bacteria (in this example, the pathogen is Serratia marcescens) the piRNAs become stronger and the sensor is silent, even at 25°C.
A link to human health?
“I think these results are interesting because they might relate to a curious phenomenon in humans known as the “hygiene hypothesis,” said Peter Sarkies, who runs the Transgenerational Epigenetic Inheritance and Evolution group.
In humans, the incidence of allergies and autoimmune diseases is known to be much higher in developed countries such as Europe and America than in poorer parts of the world. This is thought to be due to the much cleaner environment in these countries, which means that children are less exposed to pathogens. This causes their immune system to attack innocuous stimuli such as pollen and peanut butter instead!
“We think that worms and humans evolved under conditions where they were constantly exposed to pathogens. In some ways they might therefore be less well adapted to super-clean environments. Such as the laboratory in the case of worms and, perhaps, the relatively sterile environment humans in the developed world now inhabit. So maybe a little bit of dirt now and then might not be a bad thing!” said Peter Sarkies.
Tony Belicard, the postdoctoral researcher who carried out many of the experiments in this study, has just left the LMS to train to become a science teacher. We wish him all the best!
The full article The piRNA pathway responds to environmental signals to establish intergenerational adaptation to stress by Tony Belicard, Pree Jareosettasin and Peter Sarkies is published open access in BMC Biology.
This news piece was written by Dr Peter Sarkies, Head of the Transgenerational Epigenetic Inheritance and Evolution group.
