 How ADP plays to its strengths in energy regulation
How ADP plays to its strengths in energy regulation
AMP-activated protein kinase (AMPK) can be thought of as a biological transistor – transforming multiple molecular inputs into a physiological output, working as both switch and biochemical amplifier. When cellular energy levels are low, AMPK opens up energy-producing pathways and closes energy-consuming ones. It is crucial to metabolism, and problems with AMPK function have been been implicated in type 2 diabetes, obesity and cancer.
The prevailing dogma surrounding regulation of AMPK has been that AMP binding to the enzyme switches the output to ‘on’. It has been proposed that it does this in two independent ways. First, it directly activates the enzyme ‘allosterically’ (the kinase is ‘AMP-activated’), which means that it modulates the conformation and function of the enzyme. Secondly it protects AMPK against dephosphorylation – removal of a phosphate ‘safety key’ – by protein phosphatases. This step is crucial, as removing that safety key renders the enzyme inactive, a discovery made by researchers in the CSC Cellular Stress Group.
Now, working with a group from the National Institute for Medical Research, they have published new findings in the journal Nature making sense of how AMPK functions in the complex chemical environment of the cell, where levels of AMP are dwarfed by those of related adenosine diphosphate (ADP) and adenosine triphosphate (ATP). Both of these molecules compete with AMP when binding to AMPK, but the difficulties in investigating the physiological consequences of this competition has meant that previous work has been limited to investigations of AMP binding.
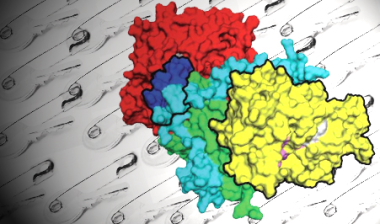
In this new work, the team has shown that the common biological molecule NADH binds to AMPK with a change in its fluorescence signature, and they have used this to investigate the strengths of binding of the three adenosine phosphates (or adenine nucleotides) to AMPK. They also present results showing how active AMPK is when ATP and ADP are bound, along with new molecular structures. The picture this new study paints is that ADP binding is an important phenomenon, and helps to explain how AMPK is regulated in the face of relatively high levels of ATP.
ATP, biology’s ‘universal battery’ (see Universal Battery) is present in cells at a concentration at least a hundred times the level of AMP, mainly in the form MgATP – associated with magnesium ions, the positive charge on which cancels out the negative phosphates. The prevalence of MgATP could spell disaster for AMPK function, because ATP is able to occupy binding pockets on the kinase normally occupied by AMP, without either activating it physically or protecting the phosphate safety key being removed. ADP, which is present at levels ten times lower than ATP, is however able to protect against inactivation of the kinase.
Universal Battery
ATP is often called the ‘universal battery of biology’, because when the bonds between the three phosphate groups are broken, a biologically-useful amount of energy is released and used to drive other chemical reactions needed for life. ATP minus one phosphate makes ADP and energy, and ADP minus one phosphate makes AMP and more energy. Like a rechargeable battery, AMP can then be ‘recharged’ by adding phosphate groups to make ATP; ultimately this comes from food and respiration.
Despite being less prevalent, ADP binds to AMPK about 10 times more strongly than MgATP. In effect, the tighter binding of ADP cancels out its 10-fold lower concentration relative to ATP, allowing the two nucleotides to compete on a level playing field. The researchers show that each AMPK molecule has three nucleotide binding pockets and make the important finding that it is binding to only one of these, termed site 3, that determines AMPK activity. When ATP is bound at site 3 AMPK adopts a conformation that allows phosphatase enzymes access to the critical phosphorylated residue – the phosphate ‘safety key’. However, when ADP is bound at this site, this residue is buried within the molecule, preventing the safety key from being removed and maintaining the kinase in an active state.
The new work gives us a more realistic picture of the cellular machinery underpinning metabolism, showing us that it is regulated in a more subtle manner than previously thought.
This research appears in Nature
Reference:
Xiao, B., Sanders, M. J., Underwood, E., Heath, R., Mayer, F. V., Carmena, D., Jing, C., Walker, P. A., Eccleston, J. F., Haire, L. F., Saiu, P., Howell, S. A., Aasland, R., Martin, S. R., Carling, D., Gamblin, S. J. (2011). Structure of mammalian AMPK and its regulation by ADP. Nature, in press.
