By Helen Figueira
April 13, 2016
Time to read: 6 minutes
Deborah Oakley
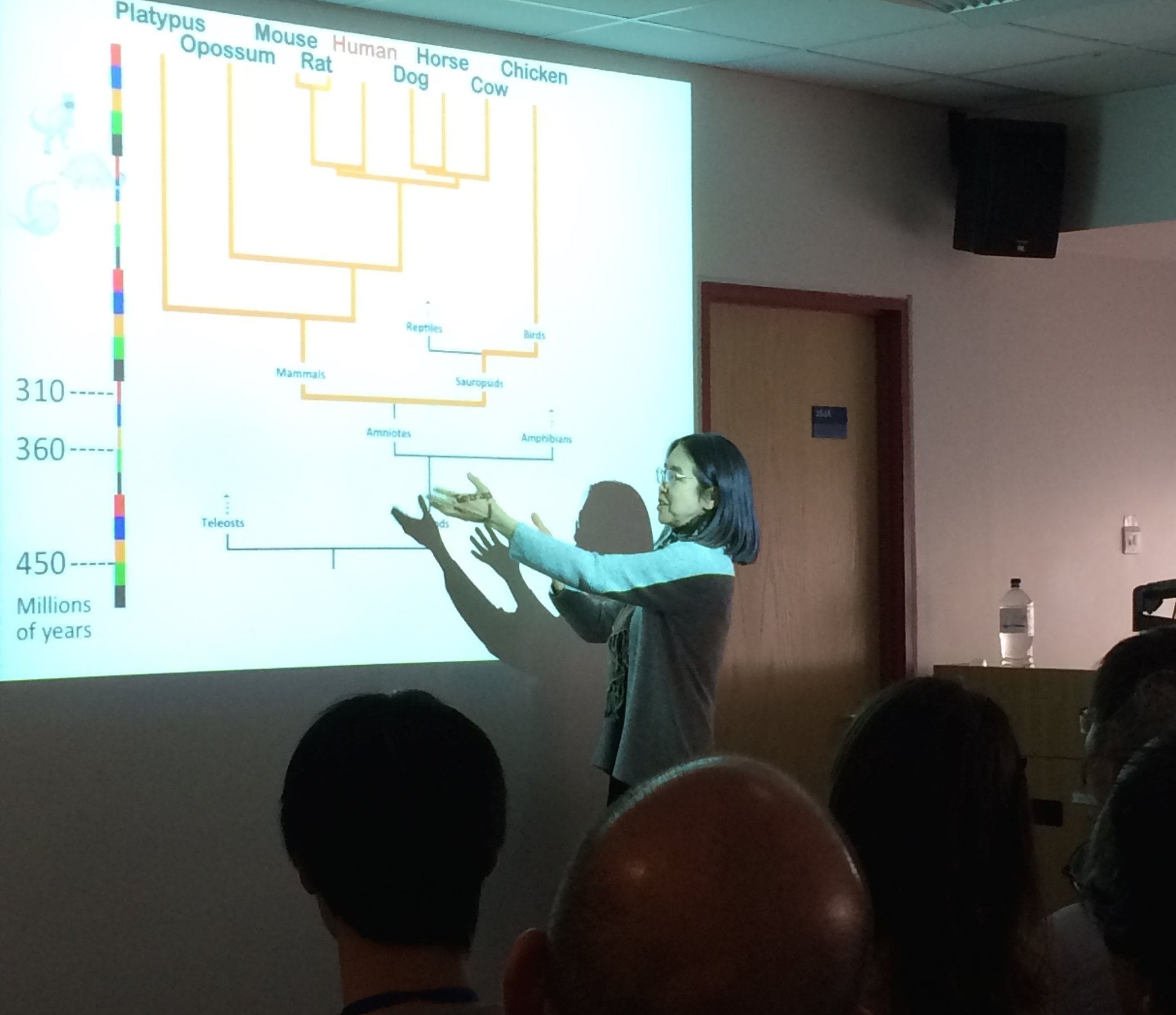
“This is one of the biggest mysteries to come from the genome era,” said Ting Wu, a professor of genetics at Harvard Medical School. She was discussing long stretches of DNA that are identical in people, mice and rats, and which have remained unchanged since our ancestors roamed the Earth around 300 to 500 million years ago. “The mystery is how these sequences resist change.”
DNA typically changes over time as species evolve, and DNA of different species evolves in different ways. So the discovery, in 2004, that almost 500 long sections of our DNA have avoided such changes took many geneticists by surprise, said Wu.
It suggested that these sequences, called ultra-conserved elements (UCEs), are vital to life. But scientists don’t fully understand what they do, or how they have survived millions of years of evolution unscathed. In fact, despite a decade of research, there has been virtually no progress in the quest to identify their origins, according to a review written by Boris Lenhard, of the MRC’s Clinical Sciences Centre (CSC), and others.
Wu thinks that she may have some of the answers. She visited the CSC this week to update scientists on her ideas, and her talk drew an eager crowd, with the audience lining the walls, sitting on tables and even on the floor.
Wu explained that her group proposes that UCEs play a role in keeping our DNA healthy. To fit inside each cell, the long strands of DNA are tightly wound up and stored in compact structures called chromosomes. Scientists know that chromosomes can pair up. Wu said that her laboratory is challenging convention by suggesting that this happens more often than was previously thought. And she thinks this may provide an answer to how UCEs have survived untouched for so long.
“We need to be prepared, every day, to have the whole model completely wrong,” Ting Wu
When two chromosomes pair up adjacent UCEs come together. According to Wu’s model, each one checks to see if the other has been altered. If damage is detected, the UCEs trigger a response that ultimately leads to their destruction. Only intact UCEs remain.
Perhaps more importantly, Wu’s model suggests that UCEs also survey other stretches of our DNA for damage. Such damage can occur when a cell makes a copy of its DNA: for example, it may make an extra copy of one gene or miss out another. Even minor mistakes can cause diseases, such as cancer. Wu proposes that UCEs somehow detect these mistakes and trigger the destruction of the mutated cells. She says that if this surveillance system goes wrong, these cells are free to grow and spread unchecked.
Her team has studied databases of information about the genes of people with 50 different cancers, and those who are healthy. They noticed that people with cancer have slight differences in the way their UCEs behave.
Despite this progress, Wu emphasised that her model of UCEs is just that: a model. When new researchers join her laboratory, she tells them, “We need to be prepared, every day, to have the whole model completely wrong.”
To find out if the model will hold up under scrutiny, Wu’s team has developed ‘oligopaints’. These molecular paints allow them to take a closer look at the way in which DNA is stored inside our body’s cells.
“I don’t think I’ve had this much fun with a seminars series in a long time,” Ting Wu
Oligopaints can bind to a specific section of DNA within a chromosome. Each probe has a label that fluoresces and acts as a colourful signal to tell the researchers where in the cell the section of DNA is at any given time, along with the chromosome of which it is a part. These probes may help the group to find out more about the nature and timing of chromosome pairing.
If correct, Wu’s model hints at a radically new approach to treat and prevent disease. “It may be possible to harness ultra conserved elements and have them work at our command to protect our genomes even more,” said Wu speaking at the Genomes, Environments and Traits conference in spring last year.
The research could even play a role in helping astronauts to survive long distance, interplanetary travel. During such voyages, astronauts would be exposed to 100 to 1000 times more radiation than we experience in a lifetime here on Earth. This onslaught could mutate their DNA and potentially lead to a barrage of illnesses. However, Wu envisages that scientists may one day be able to use UCEs to limit this damage by destroying mutated cells. Harnessing the body’s built-in defence mechanism in this way would mean that illnesses associated with radiation could be prevented, instead of treated.
Wu was the first speaker in a series of talks organised by CSC postdocs. The series provides an opportunity for early career researchers to be inspired by and network with esteemed scientists whom they admire.
“It’s an honour, I’ve had so much fun,” said Wu. “I don’t think I’ve had this much fun with a seminar series in a long time. I was thinking we should get our postdocs on my campus to do this.” She added that the postdoc period is ripe with challenges, and that supportive events like this are important.
“Wu is an extremely influential person, especially since women in science are not equally represented,” said Dimitrios Polychronopoulos, of the CSC’s Computational and Regulatory Genomics group, and a member of the postdoc committee that invited Wu. “She is also involved in many public outreach activities, having participated in TEDx and Google-sponsored events. She has also recently established the Space Consortium Project and actively participates in congressional meetings in order to promote genetics research in space.”
For further information, contact:
Deborah Oakley
Science Communications Officer
MRC Clinical Sciences Centre
Du Cane Road
London W12 0NN
T: 0208 383 3791
M: 07711 016942
E: