By Helen Figueira
July 16, 2015
Time to read: 4 minutes
Susan Watts
A gene called Jarid2, may play a wider role than previously thought in co-ordinating the way that stem cells change in a developing embryo to form the specialised cells that make up our bodies.
Stem cells in the early embryo are “pluripotent”, which means they have the ability to develop into cells with any of a number of specialised functions – such as the cells that make up the brain, heart or liver.
As these embryonic stem cells (ESCs) develop, they “differentiate” into specialised cells. Understanding how this carefully choreographed process unfolds is key to making sense of what goes wrong in inherited conditions and in the spread of some cancers, and could one day lead to better treatments.
Scientists know already that Jarid2 is important in organising the healthy formation of many organs, including the neural tubes that become the brain and spinal cord, the liver, spleen, thymus and cardiovascular system. But its central role very early on in embryo development is “surprising”, according to professor Amanda Fisher, director of the MRC’s Clinical Sciences Centre (CSC), whose team published its findings today in Cell Reports.
Learning more about the role that Jarid2 plays could shed light on the molecular basis of malformations such as congenital heart defects, cleft lip and hypoplasia, or excessive cell growth in liver spleen and blood tissues, where there is already emerging evidence that Jarid2 is important.
Jarid2 is one of a group of proteins known as the Jumonji family, which together play a central role in regulating how genes are read. This new research shows that Jarid2 also controls how cells communicate between each other, and whether neighbouring cells act alone or in concert.
“The importance of this work is that we show a relation between these different elements.” Said Hakan Bagci, a postdoc in the Fisher group. “No one has ever shown a relation between Nanog, a very important ESC pluripotency factor, and Jarid2, and no one has shown a relation between Jarid2 and Wnt signaling – an important cell signaling network,“ Bagci said.
He sees the role of Jarid2 as akin to that of the conductor of an orchestra. It appears to co-ordinate the way that some of these other factors come into play in ESCs as they make the crucial move from pluripotency to differentiation, possibly by making sure that they act on developmental cues at exactly the right moment.
For example, it’s known that levels of Nanog pluripotency factor in ESCs can fluctuate. “If you take a single cell, at specific time, Nanog levels will be low. When you analyse the same cell a day later you will see that the Nanog level is high. And the following day it will be low,” Bagci said. He explained that cell biologists think that when Nanog levels are low, the cell is prone to differentiate. When Nanog levels are high, the cell is in what’s called a naïve state, and is not ready to differentiate.
“We have a very important result in the field that no one has shown before. When there is no Jarid2 around, ESCs are all Nanog high, and are unable to respond or differentiate. But if their neighbours are normal, they can help – by instructing the cells and telling them what to do.”
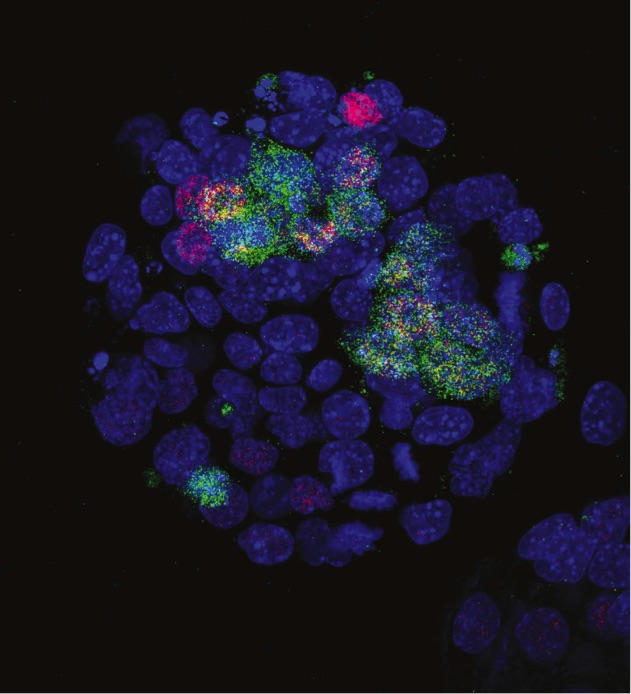
This new work may also shed some light on mechanisms behind the formation of identical twins, though this is more tentative. After fertilisation, one cell divides several times until the Inner Cell Mass (ICM) forms, which eventually gives rise to a single embryo. Cells that have no Jarid2 tend to form multiple ICMs, which may be indicative of twins.
These studies were the result of collaboration with the CSC’s transgenic facility, and used CRISPR/Cas 9 gene editing to generate new stem cell lines.
Read the Research paper here.