Dominic Withers of the MRC London Institute of Medical Sciences (LMS) appears in Nature this week with a comment piece on the journal’s latest paper on metabolism and ageing, along with co-author Colin Selman from the University of Glasgow.
By Staff Member
February 10, 2017
Time to read: 4 minutes
Dominic Withers of the MRC London Institute of Medical Sciences (LMS) appears in Nature this week with a comment piece on the journal’s latest paper on metabolism and ageing, along with co-author Colin Selman from the University of Glasgow. Read an extract and link through to the article below.
The enzyme S6K1 phosphorylates the enzyme glutamyl-prolyl tRNA synthetase to modulate metabolic activity and lifespan, revealing an atypical role for this synthetase as a target of a key metabolic signalling pathway.
Colin Selman and Dominic J. Withers
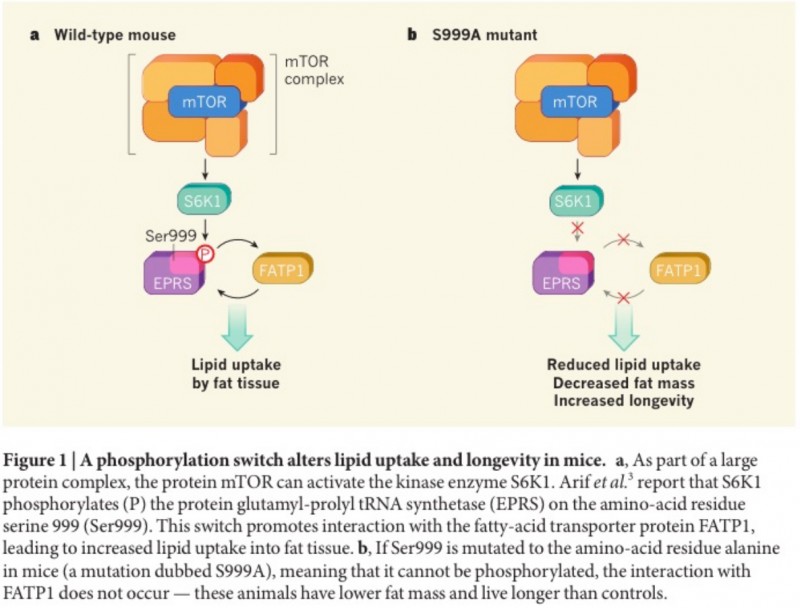
The observation a century ago that dietary restriction extends longevity in rodents led to the realization that metabolism and ageing have a close relationship. We now know that biological signalling networks that respond to nutrients and hormones modulate lifespan in a highly evolutionarily conserved manner. The protein kinase S6K1 is a key target of one such network, the mTOR (mechanistic target of rapamycin) signalling pathway, but the mechanisms down-stream of this enzyme have remained obscure. In a paper online in Nature, Arif et al. show that another enzyme, glutamyl-prolyl tRNA synthetase (EPRS), is a direct target of S6K1, and has a previously unidentified role in fat metabolism and lifespan.
Enzymes in the same family as EPRS have a key role in decoding the genome — they catalyse the binding of amino acids to transfer RNAs for transport to the cell’s translational apparatus. However, this family also fulfils many atypical roles. The research group that performed the current study previously showed in vitro that EPRS can mediate the assembly of a protein complex that attenuates the translation of certain inflammation-associated RNAs. This function depends on EPRS being phosphorylated at a particular amino-acid residue, serine 999 (Ser999), but the identity of the kinase that catalyses this phosphorylation was unclear.
In the current study, Arif et al. identified this enzyme as S6K1. Mice that lack S6K1 have previously been shown6 to display reduced fat mass, resistance to nutrient excess, delayed ageing and increased healthy lifespan (health-span) compared with control animals, and the authors set out to investigate whether EPRS is a key mediator of these effects. They generated mouse models in which EPRS phosphorylation was either blocked or mimicked by mutating Ser999 to an alternative amino acid — in the first instance, to alanine (a mutation dubbed S999A), which cannot be phosphorylated, and in the second to aspartate (S999D), which mimics permanent Ser999 phosphorylation.
Mice harbouring the S999A mutation exhibited reduced fat mass, improved metabolism and increased lifespan compared with wild-type mice, in part resembling the S6K1-deficient animals. When the S999D mutation was introduced to rescue EPRS phosphorylation in S6K1-deficient mice, a partial restoration of fat mass occurred in these animals. Together, these data suggest that EPRS phosphorylation by S6K1 plays a key part in regulating fat mass in mice, and that the absence of such phosphorylation might mediate the beneficial effects of S6K1 deletion on metabolism and ageing.
Next, Arif et al. analysed the mutants in more detail, and discovered that insulin- stimulated lipid uptake was impaired in the fat cells of S999A mice compared with control animals. The authors therefore per-formed a screen to identify components of the lipid-metabolism machinery that interact with EPRS. This showed that EPRS binds to the protein FATP1 – which mediates the uptake of lipid precursors called fatty acids into fat cells – in an insulin- and S6K1-dependent manner. These results implicate FATP1 as a key component of mTOR signalling, regulating metabolism in fat (see figure).
Although this study provides insights into metabolism and ageing, there are also some unexpected findings, unanswered questions and avenues for future investigation. Perhaps the most pressing issues relate to longevity, given the ageing human population and the recognition that ageing is a risk factor for many diseases.
Continue reading here.