Foreign DNA prompts species alert that leads to sterility
A newly published study has found that when two species mix, the sterility that often results in their offspring may be due to a surprising form of signalling between the two. Here, co-corresponding author Peter Sarkies, who leads the CSC’s Transgenerational Epigenetic Inheritance and Evolution group, summarises his findings.
By Peter Sarkies*
Nature is full of “endless forms, most beautiful”: almost anything one can imagine seems to be found in some corner of life, as Charles Darwin famously noted. Yet paradoxically, within this seemingly unending ocean of variation there exist islands of stability. Groups of organisms do not simply merge into each other along a continuous spectrum, but cluster into distinct groups, or “species”, which are very similar to each other.
“There is grandeur in this view of life, with its several powers, having been originally breathed into a few forms or into one; and that, whilst this planet has gone cycling on according to the fixed law of gravity, from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved.”

Darwin’s shells: Geological observations on the volcanic islands and parts of South America visited during the voyage of H.M.S. ‘Beagle’ by Charles Darwin. (Image courtesy of the Wellcome Image Library)
Darwin’s theory of evolution by natural selection accounts very well for variation in life forms but on its own it struggles to adequately explain why species should exist at all. Instead, Darwin’s followers came up with the Biological Species Concept, first articulated by Ernst Mayr, which suggests that a crucial step in the formation of a new species is the formation of reproductive barriers between two groups of closely related organisms such that the exchange of genetic material becomes reduced.
Eventually, these differences become so marked that any offspring produced by mating between the two groups are sterile, for example the sterile mule which is a product of a horse and a donkey. The reduced and eventually eliminated exchange of genes between these groups keeps them distinct. This must mean that there are “incompatibilities” between the two genomes, such that when they are combined the organism cannot develop normally.
However, how these incompatibilities arise or even exactly what they are is largely unknown. Thousands of genes will have differences between the two parent species and a hybrid, which will be an exact 50:50 mixture of the parent genomes, will have both versions of all of these genes. The regions that interact negatively to result in a sterile hybrid will involve only a small number of these genes, so picking these out is very difficult. Thus one approach to understanding speciation is instead to introduce small parts of the genome of one species into the genome of another, to try to see which parts result in incompatibilities.
This kind of approach is possible using model organisms that can be manipulated easily in the lab. In this study, Zhongying Zhao and his laboratory made nematode worms of a species called Caenorhabditis nigoni and introduced small regions of the X chromosome from a closely related species, Caenorhabditis briggsae. They isolated two different “foreign” regions that caused sterility.
In collaboration with Zhongying, we looked for differences in how genes are regulated that might explain the incompatibility. We discovered that small regulatory RNAs, which seek out genes and target them to be shut down, are possible culprits in this effect: the sterility was associated with an overproduction of a type of small RNA molecule found in the germline of nematode worms. The small RNAs that were overproduced were targeted predominantly against genes involved in the production of sperm, and indeed we saw that genes involved in sperm production were down-regulated and the sperm cells failed to form normally in affected individuals.
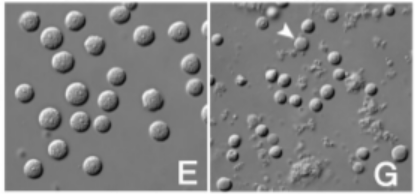
Sperm defects in hybrid animals. The sperm are smaller and there is some cell debris surrounding these, suggesting some of the cells have died. (Image Credit: Dr Zhongying Zhao via Genome Research)
What was especially interesting, and very surprising, was that the small RNAs that caused this phenomenon were not coming from the region of the chromosome that came from the other species! Indeed, we saw the same effect for two different insertions of foreign DNA into different regions on the X chromosome. This suggests that the foreign DNA is being “sensed” by the recipient, and that this is leading to sterility through suppression of spermatogenesis genes. In other words, the affected animals switch off reproduction in response to the invasion of DNA from another species.
Our work provides clues as to what might happen at the very early stages of speciation: regions of DNA, particularly on sex chromosomes, might acquire sufficient divergence to be recognised as foreign when introduced into the other species and trigger the overproduction of small RNAs that we observed. Since we induced general responses by the introduction of quite small regions of DNA, these responses might also be expected to occur during the construction of genetically modified organisms, where a small number of genes from one species are introduced into another, which could have implications for this technology.
We don’t yet know how the foreign DNA is being sensed in the first place, and how this signal is transduced to result in increased production of small RNAs. Neither do we know if the same response occurs when distinct regions from any chromosome are introduced, or whether the response is specific to the X Chromosome? We are continuing our collaboration with the Zhao laboratory to gain new insights.
*co-corresponding author on 18 May paper in Genome Research
Stress on the mind
A new article reviews evidence linking psychosocial stress and inflammation in the rodent brain. Here, co-author David Bonsall, a postdoc in Oliver Howes’ Psychiatric Imaging group, summarises his findings.
By David Bonsall**
Over the past 15 years, a number of animal studies have looked at how exposure to psychosocial stress, primarily through altering an animal’s environment, can impact on behaviour and physiology. This is of particular interest to researchers within the CSC’s Psychiatric Imaging group because in humans, exposure to stress in early-life has been associated with a greater risk of developing a mental illness such as schizophrenia later in life.
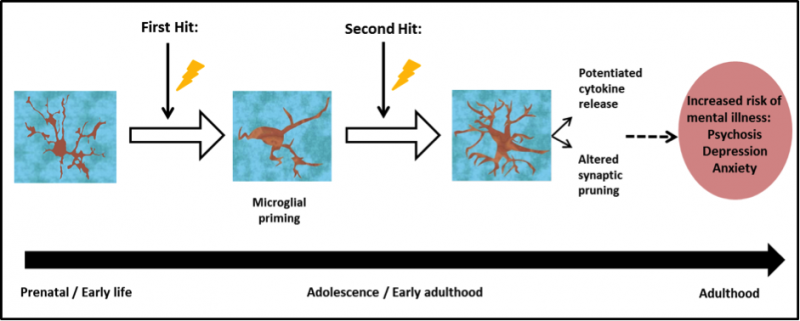
The ‘two-hit’ hypothesis: exposure to prenatal/early-life stress (lightning bolt) may act to prime microglia within the brain so that a subsequent challenge later in life, either in adolescence or adulthood, invokes an exaggerated microglial response, leading to an increased risk of developing a mental illness.
One of the major pathways through which stress can interact with and affect the central nervous system is through stimulating microglia, the primary immune cells of the central nervous system, resulting in pro-inflammatory signals and subsequent neuroinflammation. In a study published last year, our group demonstrated a link between neuroinflammation and increased risk of psychosis, with greater microglial activity found in patients with schizophrenia and those at ultra-high risk of psychosis.
The review article, published last month as part of a special Immune edition of Psychopharmacology, collates data from across 18 animal studies, to evaluate the effects of different psychosocial stressors on microglial activity. We found that a range of psychosocial stressors, including social isolation, chronic restraint and repeated social defeat paradigms (where one male forcibly takes a dominant role over all other males in the cage), in rats, mice and gerbils led to increased signals of a marker for microglia called Iba-1 across several areas of the brain.
While the majority of these studies looked at the impact of stress on adult rodents, a subset focused on the effects of prenatal stress. In these cases, maternal stress exposure during pregnancy had long lasting implications for the offspring, which when studied as adults, still showed increased Iba-1 staining in some areas of the brain such as the hippocampus, which is involved in memory formation.
The authors note that these prenatally stressed animals also showed an exaggerated immune response when subsequently presented with a second stressor – in this case by injecting a toxin later in life. This sensitised response supports the “two-hit” hypothesis of microglial priming, which suggests that a subsequent challenge in adolescence produces an exaggerated immune response and can lead to altered brain function, possibly through changes in synaptic pruning. This is the process by which microglia “prune” damaged or infected neurons – which helps keep our brains healthy. Excessive pruning, or changes in pruning can cause problems, possibly predisposing individuals to a greater risk of developing mental illness.
The importance of psychosocial stress as a primer of microglial activity has only recently started to be explored. Given the findings of this review, the authors suggest that in the future the pathways of inflammation may prove useful therapeutic targets.
