By Jenna Stevens-Smith
July 13, 2018
Time to read: 3 minutes
Research published in Cancer Cell has identified a new therapeutic target for inflammation-driven cancer. PTBP1 suppresses the secretion of inflammatory factors by senescent cells without affecting the positive effects of senescence, such as suppression of tumour growth and formation.
Senescence is a process which stops the division of old, damaged or dangerous cells. For some time, we have known that senescence plays a role in age-related diseases such as osteoporosis, cataracts, Alzheimer’s disease and cancer.
In cancer senescence has a paradoxical role. On one hand, oncogene-induced senescence imposes a stable growth arrest in premalignant cells and is a potent barrier to the formation of tumours (tumourigenesis). On the other hand, inflammatory factors produced by senescent cells contribute to the promotion of tumour growth. Inflammation is known to fuel cancer, a phenomenon we have known since the start of the 19th century. A low grade of chronic inflammation has also been shown to contribute to many age-related pathologies.
In recent years it has become apparent that senescent cells are a therapeutic target, While the strategy of choice has been the selective elimination of senescent cells (senolysis), there might be situations in which a more selective approach could be used.
In that respect, there has been a lack of understanding in the research as to whether we could target the negative consequences of senescent cells without effecting the positives. The paper published in Cancer Cell in July by the MRC LMS Cell Proliferation Group, headed by Professor Jesus Gil, investigates just this, answering the question: can we target the inflammatory Senescence-Associated Secretory Phenotype (SASP) and reduce the detrimental effects of this without hampering positive effects?
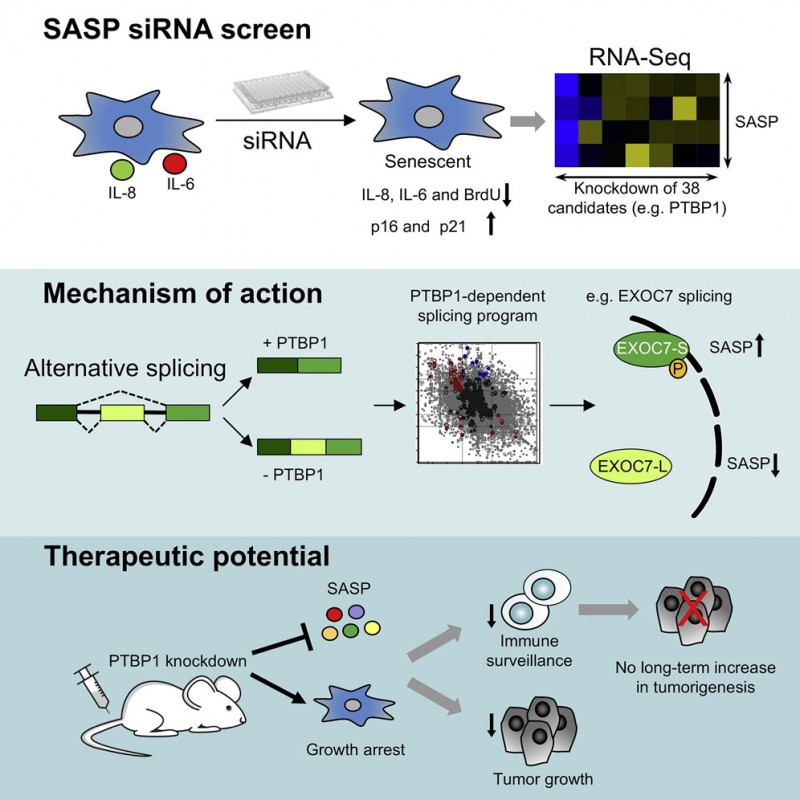
The acquisition of a senescence-associated secretory phenotype (SASP) fuels inflammation and can promote tumour progression. There are a few mechanisms that SASP is controlled by that were already known to researchers, but most of these had been found to not hold the potential to become effective therapeutic interventions.
With this in mind the researchers knew they had to look elsewhere and find new SASP controlling targets that they could modify. Within this research they screened the druggable genome and identified 50 potential new candidates. In this Cancer Cell paper, they analyse in detail one of these targets, splicing factor PTPB1.
The research has added great understanding to the mechanism of the PTPB1 gene, a biologically interesting mechanism as it connects splicing, a basic cellular process, with SASP regulation. Inhibiting PTPB1 allows for the specific suppression of the SASP, inhibiting the production of inflammatory factors, without affecting other properties associated with senescence in a way that has therapeutic potential.
Speaking to Professor Jesus Gil he discussed the findings of the paper:
“The fact that we can target the SASP and suppress the negative effects of senescence without deterring from its positive effects is the most interesting outcome of this paper. In increasing the pool of targets to regulate the SASP, we have opened up many possibilities for future basic and applied research.”
Following these findings, the team now wants to explore how splicing controls senescence and the therapeutic potential of inhibiting the SASP.
PTBP1-Mediated Alternative Splicing Regulates the Inflammatory Secretome and the Pro-tumorigenic Effects of Senescent Cells by Athena Georgilis, et al is published in the journal Cancer Cell.