
Group Members
Funding


“Senescence influences multiple biological processes including ageing and cancer.”
Cellular senescence is a stress response induced by oncogene activation, chronic inflammation, telomere erosion or DNA damage. Senescent cells activate a dynamic transcriptional programme leading to stable cell cycle exit, chromatin reorganization, metabolic reprogramming and the establishment of a senescence-associated secretory programme (SASP). Acute induction of senescence is a physiological response that limits fibrosis and acts as a potent tumour suppressor mechanism. However, the abnormal accumulation of senescent cells contributes to ageing and many diseases, including cancer. Recently, strategies aimed to selectively eliminate senescent cells (senolytic therapies) have been shown great promise for the treatment of a wide-range of diseases.
The goal of my research program is to elucidate the molecular mechanisms that implement and regulate senescence. We intend to exploit this knowledge to devise novel strategies that target senescence in age-related pathologies, especially cancer. To investigate senescence, we combine high-throughput screens and mechanistic analyses in cell culture, with in vivo analyses in mouse models. We then analyse clinical samples to uncover the relevance of our observations for human disease.
My research programme has two overarching aims: (1) Uncover novel (epi)genetic mechanisms controlling senescence. We will use functional (CRISPR, RNAi and drug) screens, proteomic and genomics to identify trans factors and cis elements that control senescence and elucidate how they function. (2) Investigate how the SASP mediates the effects of senescence. We will perform screens to discover regulators and functions of different subsets of the SASP (e.g. pro- inflammatory or fibrotic) and use novel mouse models to uncover the roles of the SASP in ageing and disease. Connecting both aims, we will search for vulnerabilities of senescence. We are actively collaborating with several companies to pursue novel ways to target senescence cells as a direct result of our research. By better understanding how senescence is regulated and implemented, we expect that our research will inform therapies that harness senescence in cancer and ageing.
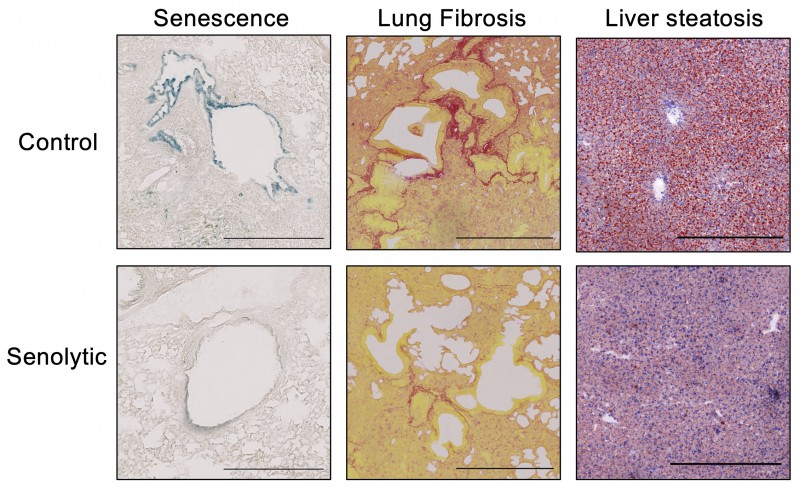
Treatment of old mice with a senolytic drug results in reduced senescence indifferent tissues (SA-β-Gal staining of lung sections, left) and reduction of senescence-associated diseases such as lung fibrosis (Picrosirius red staining, middle) or liver steatosis (Oil O red staining, right).
Selected Publications
McHugh D, Sun B, Gutierrez-Muñoz C, Hernández-González F, Mellone M, Guiho R, Duran I, Pombo J, Pietrocola F, Birch J, Kallemeijn WW, Khadayate S, Dharmalingam G, Vernia S, Tate EW, Martínez-Barbera JP, Withers DJ, Thomas GJ, Serrano M, Gil J. (2023). COPI vesicle formation and N-myristoylation are targetable vulnerabilities of senescent cells.
Nat Cell Biol. 25(12):1804-1820. doi: 10.1038/s41556-023-01287-6. Epub 2023 Nov 27.
Guerrero A, Innes AJ, Roux PF, Buisman SC, Jung J, Ortet L, Moiseeva V, Wagner V, Robinson L, Ausema A, Potapova A, Perdiguero E, Weersing E, Aarts M, Martin N, Wuestefeld T, Muñoz-Cánoves P, de Haan G, Bischof O, Gil J. (2022) 3-deazaadenosine (3DA) alleviates senescence to promote cellular fitness and cell therapy efficiency in mice.
Nat Aging. Sep;2:851-866. doi: 10.1038/s43587-022-00279-9. Epub 2022 Sep 13.
Calimport SRG, Bentley BL, Stewart CE, Pawelec G, Scuteri A, Vinciguerra M, Slack C, Chen D, Harries LW, Marchant G, Fleming GA, Conboy M, Antebi A, Small GW, Gil J, Lakatta EG, Richardson A, Rosen C, Nikolich K, Wyss-Coray T, Steinman L, Montine T, de Magalhães JP, Campisi J, Church G. (2019). To help aging populations, classify organismal senescence. Science 366, 576-578, ISSN: 0036-8075
Guerrero A, Herranz N, Sun B, Cardiac glycosides are broad-spectrum senolytics, Nature Metabolism 1, 1074–1088 doi:10.1038/s42255-019-0122-z
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. (2019). Cellular senescence: defining a path forward. Cell 179, 813-827, ISSN: 0092-8674
Georgilis A, Klotz S, Hanley CJ, Weirich B, Morancho B, Herranz N, Leote AC, Carroll T, Dharmalingam G, Wee KB, Mellone M, Guccione E, Arribas J, Barbosa-Morais NL, Heikenwalder M, Thomas GJ, Zender L and Gil J. (2018). PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell 34, 85-102.
Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martínez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ, Gil J. (2015). mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nature Cell Biology 17(9), 1205-1217.
Acosta JC, Banito A, Wuestefeld W, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Mindaugas A, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman G, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J. (2013). A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature Cell Biology 15(8), 978-990.
Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, d’Adda di Fagagna F, Bernard D, Hernando E, Gil J. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133(6), 1006–1018.
