By Sophie Arthur
April 20, 2018
Time to read: 6 minutes
A recent feature in Imperial news, Feed or flee: the brain cells that tell us when to eat and when to run away, highlights the work of Professor Dominic Withers, Head of Metabolic Signalling at LMS. These studies examined the relationship between feeding behaviour and emotional state. We met with Dominic to find out more about the role optogenetics is playing in his research and how he is using it alongside the other techniques in his lab.
Optogenetics is one of the tools Professor Withers’ group use to map the brain circuits involved in feeding and other related behaviours. In their most recent paper (Viskaitis, et al.) they were investigating the effects of anxiety and feeling externally threatened on feeding. Optogenetics, which has been developed in various laboratories over the last decade and a half or so, has won great accolades as an innovative and scientific research progressing technique including Science Magazine Method of the Year 2010, and the Brain Prize in 2013 for the researchers who developed the technique. This methodology enables researchers to activate or inhibit specific neurons in intact and freely moving animals.
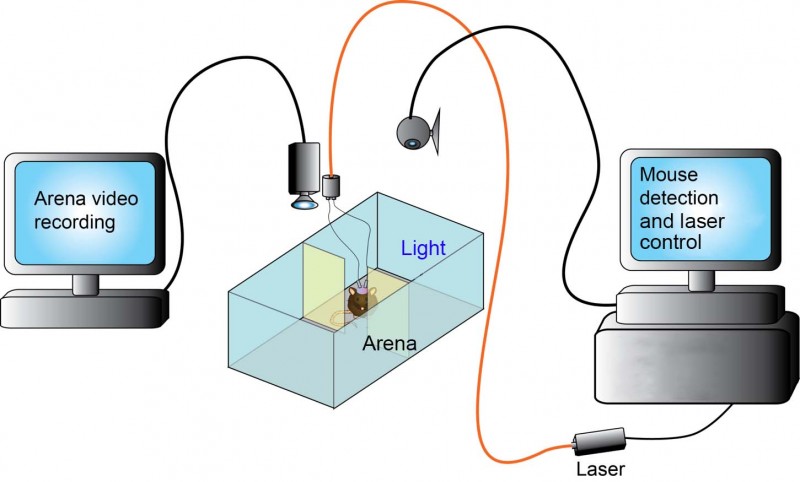
Why optogenetics/How does optogenetics work?
In the past when researching neuron activity, you could place an electrode into the brain and stimulate neurons. With this method, you were never sure exactly what neuronal type you were stimulating and would analyse an area of the brain as opposed to targeting specific neurons. Optogenetics, when combined with methods permitting cell-specific expression, allows you to target the activation or the inhibition of a particular type of neuron in a brain region with precise spatial and temporal control. The mice express opsins (light-sensing molecules) which react to light. This characteristic means that by activating the opsin neuron, activity can be inhibited or stimulated. The light comes from either a fibre optic cable directly into the brain and connected to a laser or more recently there are infrared ways of getting light to activate neurons allowing external stimulation of neuronal activity.
How does optogenetics compare to past techniques
In optogenetics you simply change the excitability of a cell, you don’t get rid of it completely or chronically manipulate it as was achieved with more traditional cell ablation or gene targeting approaches. This allows a more natural ability to dissect, in intact animals, the acute effects of manipulating a specific circuit. There is increasing sophistication with the virus and recombinase mice to work on multiple circuits. Optogenetics allows you to see these affects in real time. For example, with the light source off a neuronal population is behaving normally but when the laser is switched on the excitability of the neuronal population is changed and you may elicit or inhibit a particular behaviour.
Techniques that complement optogenetics
We are one of first groups in the UK to use miniature fluorescence imaging cameras, that can be attached to a mouse’s head and allow the recording of the natural activity of neurons of the mice. When using these cameras, which in our case were made by Inscopix, you aren’t manipulating the activity but you are recording the neural activity and are able to analyse it while mice are freely moving around and behaving naturally.
We also utilise chemogenetics[1], a method that allows us to chemically control circuits, which can be used alongside optogenetics. For our chemogenetics work we specifically use DREADDs (Designer Receptors Exclusively Activated by Designer Drugs)[2]. These engineered receptors are not responsive to endogenous ligands but can be activated by low concentrations of a specific drug-like molecule that was designed to have no effects itself in the absence of the DREADDs. In the same way as opsins, the DREADDs can be expressed using viruses or transgenically in mice and activated by injecting the drug or putting it in the animal’s drinking water. As this approach does not require a laser and fibre-optic cables different sorts of assays can be used. The DREADDs and other chemogenetic tools can be used for longer-term studies over days rather than the shorter-term optogenetic studies.
What is next?
We have a paper coming out with Dr Mark Ungless, Senior Lecturer at Imperial College London, about the effect of stimulating midbrain dopamine neuron activity on salt consumption, utilising both optogenetics and chemogenetics to excite and inhibit dopamine neurons. We continue to look into feeding and behaviour focussing on the hypothalamus, at the base of the brain, and the higher neural circuits that talk to the hypothalamus to make you eat or stop you eating. Being able to use optogenetics to disentangle chemically defined populations of neurons based on what neurotransmitters or what peptides they express may help us to realise the potential for developing treatments for chronic conditions like obesity in humans.
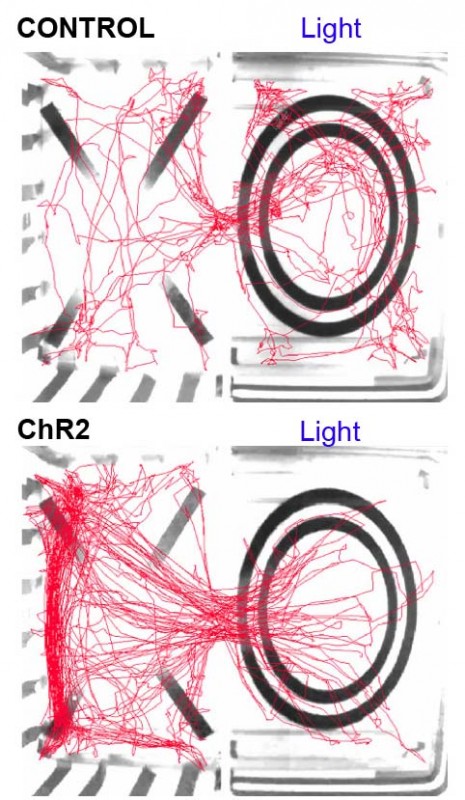
* Original video footage showing the behaviour of a mouse in the real-time place avoidance assay. When the mouse enters the right-hand side of the area with the circle, it receives optogenetic stimulation of SF1 neurons which causes the mouse to leave the area as this is an aversive stimulus. The mouse avoids the area of the arena in which it receives the laser-stimulation of SF1 neurons.
[1] Combined Optogenetic and Chemogenetic Control of Neurons https://www.ncbi.nlm.nih.gov/pubmed/26965125
[2] DREADDs for Neuroscientists https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4759656/
Figures & video copyright Withers/Viskaitis.