By Sophie Arthur
June 5, 2018
Time to read: 3 minutes
The number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014 according to the most recent statistics from the World Health Organization. Research by Professor Dominic Withers, Head of Metabolic Signalling, and Dr Mathieu Latreille, Head of Cellular Identity and Metabolism, at MRC LMS, has provided new insight into a mechanism involved in diabetes and obesity.
The paper, published yesterday in the Journal of Clinical Investigation, profiles the precise cellular and molecular mechanisms of Neuronatin (Nnat), a gene known to be involved in brain development and that has also previously been implicated in obesity.
Diabetes usually results from two abnormal processes: a failure to secrete enough insulin from pancreatic beta cells, the cells where insulin is stored and secreted, and a failure of insulin to work properly in its target tissues. Professor Withers and the team of researchers found that Nnat unexpectedly plays a role in the control of insulin processing.
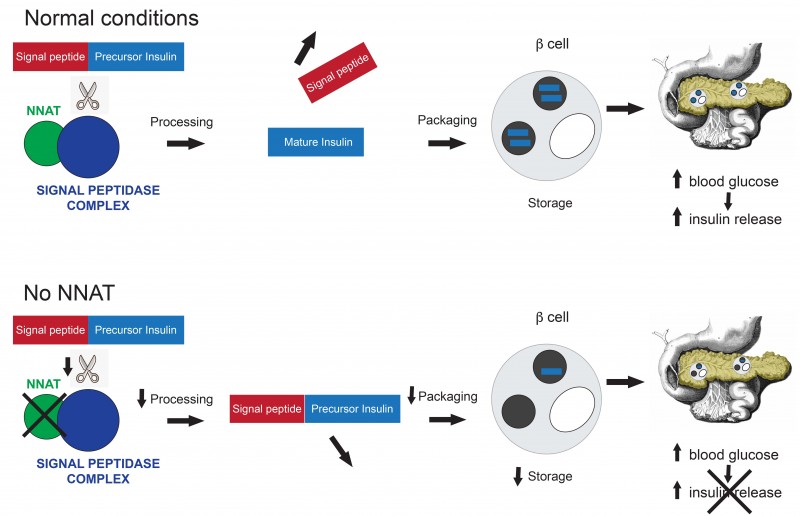
Normally, insulin is produced with a specific signal at one end that allows it to find the right destination inside the cell. Once this signal peptide is chopped off by an enzyme the insulin gets properly packaged, stored and primed to be released when blood glucose levels rise.
The researchers found that mice that do not have the Nnat gene are inefficient at this processing and have a reduced ability to store and secrete insulin resulting in the development of diabetes.
In detailed studies in cells and in the test tube, the team found that the reason behind the development of diabetes in mice was that Nnat plays a key role in the efficient removal of the signal peptide.
Within the study they also found that the levels of neuronatin inside pancreatic beta cells, were in turn regulated by the availability of glucose and nutrients. These findings suggest that Nnat is involved in properly matching insulin secretion to the levels of glucose in the blood and thus identified a new method of metabolic control.
Professor Withers shared his thoughts,
“We have undertaken a series of complex studies moving from mice lacking Nnat to detailed studies in cells to reveal a new way in which insulin secretion is controlled. While these studies have not directly focussed on human diabetes we know that Nnat is found in human beta cells and that defective processing of insulin due to other abnormalities can cause diabetes.”
Steven Millership, first author, said,
“What is particularly interesting is that Nnat is also found in a range of other tissues important for regulating metabolism such as adipose tissue and the brain and therefore the mechanism we have found may also be important for the processing, storage and secretion of other metabolic hormones.”
Future studies will look further into the role of Nnat to allow us to better understand neuroendocrine cell secretion and the regulation of energy homeostasis.
To hear more about the work of the Metabolic Signalling Group you can read our previous articles “Shining a light on metabolic signalling research” and “Neural insight into salt intake.”
Imperial College London have also published a piece about the Millership et al., 2018 paper, “How a gene linked to obesity could provide new insights into diabetes.”