By Sofia Velasquez-Pimentel
March 31, 2023
Time to read: 5 minutes
New Breakthrough in Duchenne Muscular Dystrophy Research: New compounds discovered that increase utrophin expression may provide a new therapeutic strategy for DMD.
By Sofia Velazquez Pimentel
The LMS Epigenetic Memory Group has made a significant breakthrough in the search for a therapeutic strategy for Duchenne Muscular Dystrophy (DMD), a debilitating genetic disorder that mainly affects young boys. The team developed a state-of-the-art method using dual-coloured gene reporters to model the expression of the mutated gene dystrophin and its paralogue utrophin to screen for compounds to enhance utrophin expression in developing muscle cells, as a potential therapeutic strategy for DMD.
Duchenne Muscular Dystrophy, or DMD, is a debilitating genetic disorder that mainly affects young boys, affecting one in every 3,500 to 5,000 male births worldwide. DMD is characterized by progressive muscle degeneration and weakness due to the alterations of a protein called dystrophin that helps keep muscle cells intact. These alterations in dystrophin are caused by a mutation in the Dmd gene. Although there is currently no cure, there are immense efforts in genetic and epigenetic research to discover treatments and cures for DMD.
Under Professor Amanda Fisher’s lead, the team from the LMS Epigenetic Memory Group developed a cutting-edge method using dual-coloured bioluminescent gene reporters to model the expression of the mutated gene Dmd and its paralogue utrophin (Utrn). Previous research, involving artificially increased utrophin expression in muscular dystrophy animal models has shown that utrophin upregulation can partially compensate for the lack of dystrophin. This suggested that utrophin upregulation is a potential therapeutic option for Duchenne Muscular Dystrophy.
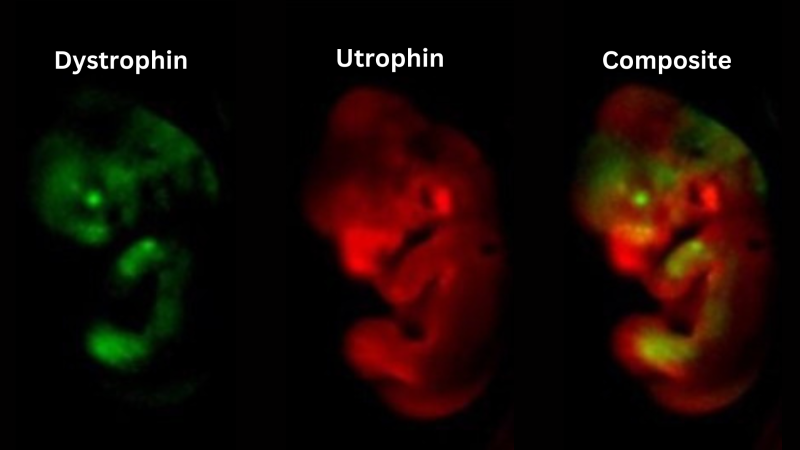 Two different luciferase enzymes, which emit light of distinct colours were inserted into endogenous dystrophin and utrophin genes, allowing both genes to be visualised within the same animal. Here we show that while dystrophin and utrophin have distinct expression profiles, both genes are expressed in the limbs of mid-gestation embryos.
Two different luciferase enzymes, which emit light of distinct colours were inserted into endogenous dystrophin and utrophin genes, allowing both genes to be visualised within the same animal. Here we show that while dystrophin and utrophin have distinct expression profiles, both genes are expressed in the limbs of mid-gestation embryos.
In their recent publication in Communications Biology, the researchers set out to discover candidate drugs that could modify chromatin-modifying drugs or signalling pathway inhibitors to enhance endogenous Utrn expression. They created a new in-vivo technique of bioluminescent gene reporters to model the expression of dystrophin and utrophin in mice, mice embryos and developing muscle cells. Using cells derived from these mice, a bioluminescent drug screen showed that treatment with different PRC2 inhibitors increased utrophin expression in myoblasts. Inhibition of ERK1/2 signalling also increased utrophin expression, and this effect was maintained as myoblasts differentiated. Furthermore, additional effects were observed when the two inhibitors were combined.
The team used a technique called Optical projection tomography to visualise utrophin expression (shown in red) in mouse embryos.
‘Our paper describes new screening tools and potential therapeutic options for the treatment of Duchenne Muscular Dystrophy’ said co-first author Dr Hannah Gleneadie. ‘Our dual colour luciferase reporter mouse line can allow the expression of both genes to be visualised in the same animal, longitudinally and throughout embryonic development. Additionally, utrophin luciferase reporter myoblasts can be used to screen for compounds which increase utrophin expression in adult skeletal muscle’.
‘Our paper describes new screening tools and potential therapeutic options for the treatment of Duchenne Muscular Dystrophy. Our dual colour luciferase reporter mouse line can allow the expression of both genes to be visualised in the same animal, longitudinally and throughout embryonic development. Additionally, utrophin luciferase reporter myoblasts can be used to screen for compounds which increase utrophin expression in adult skeletal muscle’.
– Co-first author Dr Hannah Gleneadie
The preclinical mouse model developed in this study is a valuable tool for the screening of drug candidates that can enhance utrophin expression. The findings suggest that inhibiting the PRC2 and ERK1/2 pathways can increase utrophin expression and could be a potential therapeutic strategy for DMD. The specialized molecular imaging tools developed and the findings in this publication could be pivotal to future research in monitoring utrophin expression as a therapeutic strategy for DMD.
While further research is needed to investigate the long-term effects of PRC2 and ERK1/2 in vivo and human cells, these results pave the way for exploring the role of ERK1/2 and PRC2 inhibitors for the treatment of DMD.
‘Currently, treatment with corticosteroids and assisted ventilation are the standard clinical care for DMD, although these do not address the underlying molecular biology of the disease. We have developed new animal models to better understand the dynamics between dystrophin, the failing protein in DMD, and utrophin, its potential substitute, and we have uncovered potential therapeutic opportunities based on utrophin overexpression. In contrast with other therapeutic strategies, utrophin-based treatments could offer a universal treatment to all DMD patients, regardless of their mutation in dystrophin.’
– Dr Beatriz Fernandez-Ruiz, co-first author
This research was published in Communication Biology by the LMS Epigenetic Memory Group, LMS WAPI facility, GSK, Imperial College London, and the LMS Lymphocyte Development Group.
This work was funded by the Medical Research Council and was supported by funding from the British Heart Foundation.