
David Carling
Group Members
Funding

“Our research is aimed at understanding the regulation of energy metabolism”
AMP-activated protein kinase (AMPK) is activated in response to a fall in the intracellular concentration of ATP, and has been dubbed the cell’s fuel gauge. AMPK phosphorylates a wide range of downstream targets involved in diverse pathways. Broadly speaking, activation of AMPK leads to an increase in catabolic pathways and a reduction in anabolic pathways, effects that are predicted to be beneficial in metabolic diseases. As a consequence, AMPK has attracted significant attention as a potential therapeutic target for drugs aimed at treating metabolic diseases, including obesity and type 2 diabetes.
Phosphorylation and activation of AMPK is catalysed by either LKB1 or Ca2+/calmodulin dependent protein kinase kinase 2 (CAMKK2). Inactivating mutations within LKB1 lead to a rare, dominantly inherited cancer predisposition in humans, termed Peutz-Jeghers Syndrome, whereas CAMKK2 expression is increased in human prostate cancer. These findings suggest that AMPK may play a role in human cancers. Our current focus is to understand the regulation of AMPK using structure/function analyses and to explore its physiological role using a recently developed transgenic mouse model expressing a gain-of-function AMPK mutant.
An exciting new development is our finding that AMPK activation protects against diet-induced obesity by increasing thermogenesis in white adipose tissue (WAT). Importantly, the mechanism for increased thermogenesis does not involve UCP1, but appears to utilise a calcium cycling mechanism. A key challenge for the future is to identify the cells responsible for the non-UCP1mediated thermogenesis in WAT and to characterise the molecular mechanisms controlling their growth and differentiation.
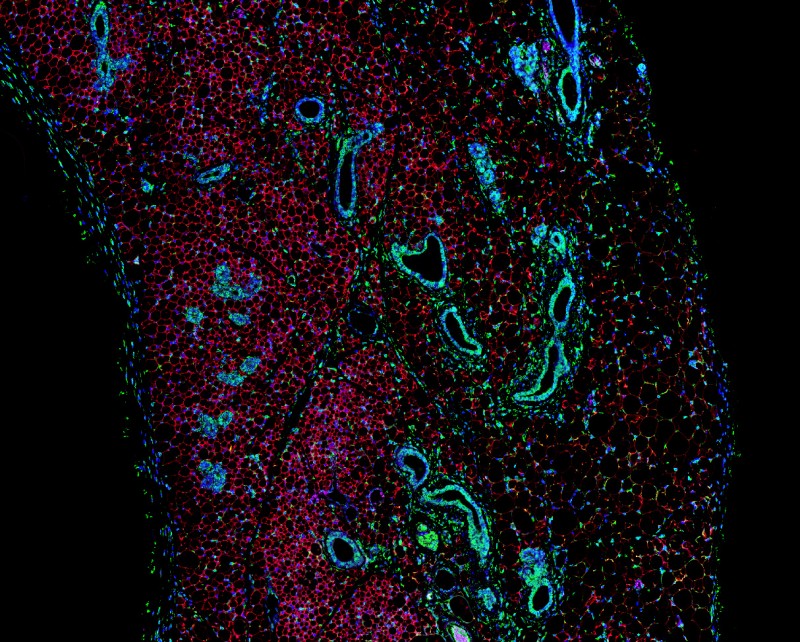
Confocal image illustrating that AMPK activation alters the morphology of subcutaneous white adipose tissue, increasing vascularisation and promoting multilocular adipocyte formation. RED- Perilipin 1 (a lipid droplet-associated protein), GREEN-tubulin (a cytoskeletal protein), BLUE-DAPI (nuclei).
BBSRC (fund Alice Pollard) and BHF (fund Sophie Newton)
Selected Publications
Penfold, L., Woods, A., Pollard, A.E., Arizanova, J., Pascual-Navarro, E., Muckett, P.J., Dore, M.H., Montoya, A., Whilding, C., Fets, L., Mokochinski, J., Constantin, T.A., Varela-Carver, A., Leach, D.A., Bevan, C.L., Nikitin, A.Y., Hall, Z., Carling, D. (2023). AMPK activation protects against prostate cancer by inducing a catabolic cellular state. Cell Reports, 42 (4), art. no. 112396.
Hope, D.C.D., Hinds, C.E., Lopes, T., Vincent, M.L., Shrewsbury, J.V., Yu, A.T.C., Davies, I., Scott, R., Jones, B., Murphy, K.G., Minnion, J.S., Sardini, A., Carling, D., Lutz, T.A., Bloom, S.R., Tan, T.M.M., Owen, B.M. Hypoaminoacidemia underpins glucagon-mediated energy expenditure and weight loss. (2022). Cell Reports Medicine, 3 (11), art. no. 100810.
Widjaja, A.A., Viswanathan, S., Wei Ting, J.G., Tan, J., Shekeran, S.G., Carling, D., Lim, W.-W., Cook, S.A. IL11 stimulates ERK/P90RSK to inhibit LKB1/AMPK and activate mTOR initiating a mesenchymal program in stromal, epithelial, and cancer cells. (2022). iScience, 25 (8), art. no. 104806.
Wilson, L., Pollard, A.E., Penfold, L., Muckett, P.J., Whilding, C., Bohlooly, M.Y., Wilson, P., Carling, D. (2021). Chronic activation of AMP-activated protein kinase leads to early-onset polycystic kidney phenotype. Clinical Science, 135 (20), pp. 2393-2408.
Pollard AE, Martins L, Muckett PJ, Khadayate S, Bornot A, Clausen M, Admyre T, Bjursell M, Fiadeiro R, Wilson L, Whilding C, Kotiadis VN, Duchen MR, Sutton D, Penfold L, Sardini A, Bohlooly-Y M, Smith DM, Read JA, Snowden MA, Woods A and Carling D. (2019). AMPK activation protects against diet induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nature Metabolism, 1, 340-349.
