“We intend to characterise the molecular mechanisms driving metabolic homeostasis in health and in metabolic pathologies such as diabetes and obesity.”
The rising prevalence of metabolic pathologies such as obesity, type 2 diabetes (T2D) or non-alcoholic fatty liver disease (NAFLD) constitutes a major health problem.
We use both computational and experimental approaches to identify and characterize new metabolic regulators relevant for human metabolic pathologies to uncover novel targets for the design of improved diagnostic and therapeutic strategies.
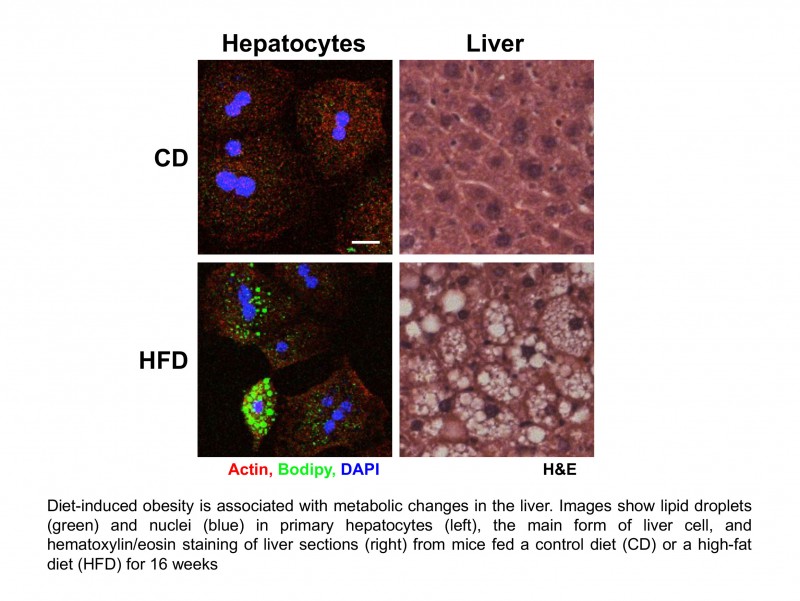
Our short-term goal is to characterize the molecular mechanisms driving obesity-associated liver disease at different stages (insulin resistance, NAFLD, nonalcoholic steatohepatitis, fibrosis or hepatocellular carcinoma) with an emphasis on the role of pre-RNA splicing and alternative splicing in this process.
Selected Publications
Guerrero A, Herranz N, Sun B, Cardiac glycosides are broad-spectrum senolytics, Nature Metabolism doi:10.1038/s42255-019-0122-z
Milona A, Massafra V, Vos H, Naik J, Artigas N, Paterson HAB, Bijsmans I, Willemsen E, Miguel-Aliaga I, Bosma P, Burgering B, Williamson C, Vernia S, Dhillo WS, van Mil S, Owen BM. (2019). ‘Steroidogenic Control of Liver Metabolism Through a Nuclear Receptor-Network‘, Mol. Endocrinology, 2019. Dec;30:221-229.
Barutcu SA, Girnius N, Vernia S, Davis RJ. (2018). Role of the MAPK/cJun NH2-terminal kinase signaling pathway in starvation-induced autophagy. Autophagy 14(9), 1586-1595. DOI:10.1080/15548627.2018.1466013.
Soltis AR, Motola S, Vernia S, Ng CW, Kennedy NJ, Dalin S, Matthews BJ, Davis RJ, Fraenkel E. (2017). Hyper-and hypo-nutrition studies of the hepatic transcriptome and epigenome suggest that PPARα regulates anaerobic glycolysis. Scientific Reports 7(1), 174.
Vernia S, Edwards YK, Han MS, Cavanagh-Kyros J, Barrett T, Kim JK, Davis RJ. (2016). An alternative splicing program promotes adipose tissue thermogenesis. eLife 5:e17672.
Tang Y, Wallace M, Sanchez-Gurmaches S, Hsiao WY, Li H, Lee P, Vernia S, Metallo C, Guertin DA. (2016). Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat Commun 7, 11365.
Vernia S, Cavanagh-Kyros J, Barrett T, Tournier C, Davis RJ. (2016). Fibroblast Growth Factor 21 Mediates Glycemic Regulation by Hepatic JNK. Cell Rep 14(10), 2273-80.
Vernia S, Morel C, Madara J, Cavanagh-Kyros J, Barrett T, Chase K, Kennedy N, Jung DY, Kim J, Aronin N, Flavell R, Lowell BB, Davis RJ. (2016). Excitatory transmission onto AgRP neurons is regulated by cJun NH2-terminal kinase 3 in response to metabolic stress. eLife 5:e10031.
Vernia S, Cavanagh-Kyros J, Barrett T, Tournier C, Davis RJ. (2016). Fibroblast growth factor 21 mediates glycemic regulation by hepatic JNK. Cell Reports 14(10), 1-8.
Thornton TM, Delgado P, Chen L, Salas B, Krementsov D, Fernandez M, Vernia S, Davis RJ, Heimann R, Teuscher C, Krangel MS, Ramiro AR, Rincón M. (2016). Inactivation of nuclear GSK3β by Ser(389) phosphorylation promotes lymphocyte fitness during DNA double-strand break response. Nat Commun 7, 10553.
Vernia S, Cavanagh-Kyros J, Garcia-Haro L, Sabio G, Barrett T, Jung DY, Kim JK, Xu J, Shulha HP, Garber M, Gao G, Davis RJ. (2014). The PPARα-FGF21 Hormone Axis Contributes to Metabolic Regulation by the Hepatic JNK Signaling Pathway. Cell Metabolism 20(3), 1-14.
Vernia S, Cavanagh-Kyros J, Barrett T, Jung DY, Kim JK, Davis RJ. (2013). Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes and Development 27(21), 2345-55.


