With more than 10% of couples affected by infertility worldwide, research into the development of germ cells – cells that eventually form the sex cells or gametes (i.e. either the female egg cell or male sperm cell) – has been gaining momentum in the past decade. As a result, scientists have acquired a much better understanding of the molecular principles of early mammalian germ cell formation, being able to generate early germ cell like cells, known as primordial germ cell (PGC) like cells (PGClCs), in a culture dish in vitro.
Until now, it has however remained impossible to initiate the formation of functional sperm or egg cells from these early germ cell like cells in vitro. Research published in Nature by Prof Hajkova, Head of Reprogramming and Chromatin at LMS, and her team provides a molecular explanation for this developmental barrier and the route that could be used to overcome it. These new findings thus pave a clear way towards the possibility of developing gametes fully in vitro.
When PCGs are first created in the mammalian embryo they are similar to stem cells, they are ‘naïve’, undifferentiated, and have not taken up their sexual identity. This means the early germ cells are the same in both male and female embryos. The key moment in further PGC development is the initiation of meiosis; during this process the amount of genetic material is halved, which eventually allows for the generation of either a mature egg or sperm cell.
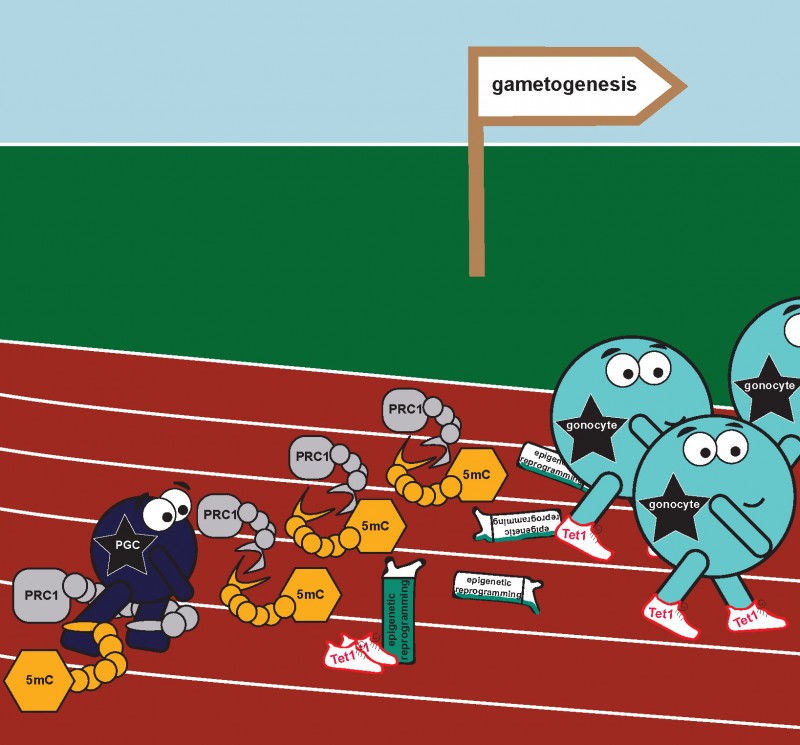
Developmental progression from early germ cells (PGC – primordial germ cells) to gonocytes capable of initiating meiosis crucially requires simultaneous release from both DNA methylation and Polycomb (PRC1) repression, and the presence of Tet1 oxygenase in both DNA methylation dependent and independent roles (see Hill et al, Nature 2018)
The newly published research explains how PGCs gain the capacity, at the molecular level, to enter into meiosis. The current work from Petra’s team and her collaborators from New England Biolabs (MA, USA), explains how the various epigenetic components (chemical modifications of DNA and of the associated histone proteins) act together at this important stage of PGC development. The key finding is that the initiation of the meiotic programme involves simultaneous deletion of two types of repressive epigenetic information: DNA methylation, responsible for modifying DNA; and the polycomb repressive system, responsible for adding specific types of chemical modifications to certain histone proteins that DNA is wrapped around.
The authors explain how this combined deletion, of DNA methylation and the polycomb repressive system, generates an epigenetically ‘naïve’ state, a state in which the DNA and its structural proteins are largely unmodified. They go on to show that this state is absolutely essential for PGCs to acquire the potential, at the molecular level, to enter into meiosis, thus progressing into the next stage of their development. These findings therfefore provide a so far missing piece of the puzzle towards our efforts to make gametes in vitro and heightens our understanding of the process which holds the key to treating infertility.
Read the full study here.
