 Senescence and Hypercholesterolaemia
Senescence and Hypercholesterolaemia
The mathematician Paul Erdös used to tell people that his colleagues had died when they left mathematics; when they actually died, he said they had “left”. It is certainly true that many of us feel defined by our jobs, and can even be at a loss when we no longer have to work.
Cells also have a finite ‘working life’, at least when it comes to cell division: after about 50 replications, they stop dividing but otherwise continue as living cells. This cellular retirement – or senescence – has its advantages in limiting the propagation of genetic copying errors that can accrue over time.
Researchers in the Clinical Sciences Centre’s Lipoprotein group were studying a disease called Autosomal Recessive Hypercholesterolaemia (ARH), which reduces a person’s ability to deal with low density lipoproteins or ‘bad cholesterol’. They noticed that skin cells from ARH patients grow slowly and go into senescence earlier than expected, so they considered whether the ARH protein – which is deficient in ARH patients due to a faulty gene – could have additional biological functions to that of dealing with cholesterol. A team from the University of California had found that the ARH protein plays role in cytokinesis, the process in cell division that splits up a cell’s membrane and fluid contents as it divides. Perhaps this held a clue to the slow growth they observed?
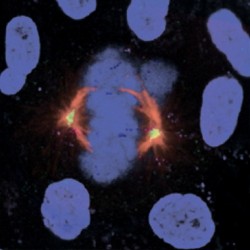
The Lipoprotein group teamed up with CSC Cell Proliferation, whose expertise in cell growth would prove valuable in making sense of what they saw. “In addition to observing premature senescence,” reports researcher Dr Xi-Ming Sun, formerly of the Lipoprotein group, “we noticed that the cellular nuclei were misshapen, and that there were problems with cell division. As well as the role in cytokinesis previously reported, we wondered whether the ARH protein might be important for chromosome duplication.” They introduced a molecular marker that is taken up during DNA synthesis, which is central to chromosome duplication, and showed that it was being taken up more slowly, explaining the slow cell growth.
In addition to this, they were able to confirm that the ARH protein is concentrated at locations in the cell crucial for cell division, specifically the mitotic spindles and nuclear membrane. It interacts with a protein called lamin B, “…a nuclear envelope protein; possibly part of the ‘mitotic matrix’ – a relatively new idea that many proteins are important for maintaining the structure of mitotic spindles,” explains Dr Sun. “Mitotic spindles are where the sister chromatids – the two cohorts of copied chromosomes resulting from cell division – line up before cells divide; it is also possible that the ARH protein is part of this mitotic matrix.” A role in mitosis could explain the reduced cell growth and premature senescence the researchers saw. But when it comes to senescence, there is the possibility that an alternative underlying mechanism could be at play.
Senescence can also be brought on sooner than usual in response to stresses, much like an incapacitating injury can force early retirement. Research published by a group at the University of Vermont shows that along with other proteins, ARH becomes partly phosphorylated in response to radiation damage, limiting its ability to function. “If the protein plays a role in DNA damage response, this could also explain the premature senescence exhibited by cells deficient of ARH,” suggest the CSC authors.
Their findings could also have important clinical relevance, because cellular senescence occurs during the development of atherosclerosis, the thickening of arteries by bad cholesterol that can lead to cardiovascular disease including heart attack.
“We already knew that a deficiency of ARH protein can lead to atherosclerosis through an inability to process ‘bad’ cholesterol,” explains Head of Lipoprotein Professor Anne Soutar, “…but our finding that ARH is also important in senescence, which is an important contributing factor in the development of atherosclerosis, has not been previously explored.”
Their findings help us to understand how the body deals with the fat in our diets, and perhaps also identify the play of a subtle mechanism that helps determine how cells replicate and when they eventually retire.
SJ
Sun X-MM, Patel DD, Acosta J-CC, Gil J, Soutar AK (2011) Premature senescence in cells from patients with autosomal recessive hypercholesterolemia (ARH): Evidence for a role for ARH in mitosis. Arteriosclerosis, thrombosis, and vascular biology, in press.
http://dx.doi.org/10.1161/ATVBAHA.111.232223
