 CSC team pioneers novel imaging technology
CSC team pioneers novel imaging technology
Novel imaging technologies provide increasingly powerful ways to probe the behaviour of cellular molecules in living organisms and have the potential to enhance animal welfare. “We hope the novel technologies we’re developing could help us better understand how disease develops” reveals Alex Sardini (Cellular Stress Group), “and fewer animals will be required for experiments in the future.”
Alex and his collaborators are harnessing optical fluorescence, in which molecules emit light at one wavelength when illuminated with light at another, shorter wavelength. This has proved to be a powerful tool for investigating biological processes inside living cells. A refinement of the basic technique is to use two fluorophores which interact when they are brought within a few nanometres of one another – called Förster Resonant Energy transfer (FRET). These fluorophores can be attached to proteins, and so the technique can be used to detect when proteins come together or move apart, giving a clue to the biological processes occurring.
To date however, these powerful approaches have not realised their full potential because they have mainly been applied to the study of isolated cells. If such ‘bio-reporters’ could be expressed in cells in vivo, and their fluorescence signals read out, cell functions could be directly monitored in the context of the whole organism. This could have a tremendous impact on our ability to understand the mechanisms of disease and to develop and test new therapies.
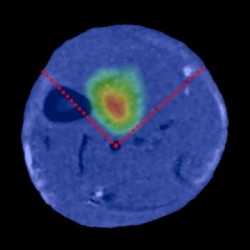
The researchers are developing a platform technology, combining FRET detection with tomographic reconstruction, to non-invasively monitor cellular processes in vivo with sufficient spatial localisation so to be able to determine in which organs these processes reside.
“This is a strong collaborative project, supported by the Wellcome Trust, between researchers at the CSC (Dan Stuckey, Alex Sardini and Jo Hajnal), UCL (Vadim Soloviev and Simon Arridge) and Imperial College (James McGinty and Paul French)”, says Jo Hajnal (Imaging Physics and Engineering Group), “It is extremely challenging to measure fluorescence signals in biological tissue due to the strong scattering that light undergoes. We circumvent this problem by using ultra-short pulses of laser light and measuring the time variation of the resulting fluorescence signals using novel detector technology, rather than simply measuring the fluorescence intensity.” By applying their imaging techniques at timed intervals the group has mapped their bio-reporter signals within mice.
This capability has huge potential in long-term experiments and is likely to allow reduced animal usage for the mandatory tests of efficacy and toxicity required during the development of new drugs. “And not only that,” adds Alex, “the technology could be used to explore disease mechanisms and track disease progression, for example, by expressing FRET probes to monitor cell signalling under live conditions.”
Globally there is a race going on to find new ways to image fluorescent probes in live animals. “Although other groups across the world have published various steps towards this,” says Jo Hajnal, “our paper is the first example showing a technology that has made it possible to localize and detect the activity of a FRET probe in vivo.”
BM
*McGinty, J., *Stuckey, D. W., Soloviev, V. Y., Laine, R., Wylezinska-Arridge, M., Wells, D. J., Arridge, S. R., French, P. M. W., Hajnal, J. V., Sardini, A., Jul. 2011. In vivo fluorescence lifetime tomography of a FRET probe expressed in mouse. Biomed. Opt. Express 2 (7), 1907–1917. (*Joint first authorship)
