Deborah Oakley
Researchers exploring the complex structure in which our DNA is stored inside our body’s cells have demonstrated that this structure depends crucially on a protein called ‘Hira’.
Hira acts like a chaperone, moving into place the proteins that make up the structure’s foundations. Without it, the structure could not be properly assembled, leaving the DNA exposed to potential damage.
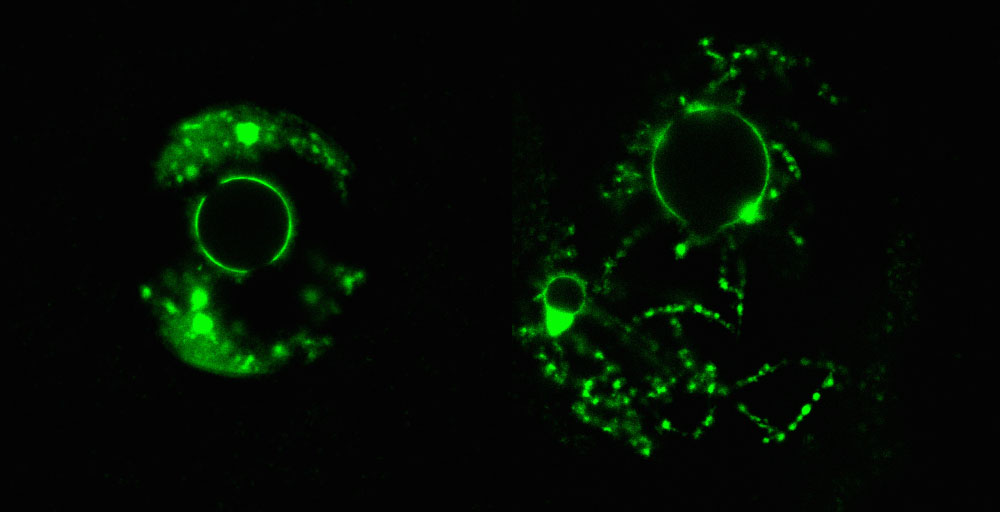
Cellular chaperone Hira is key to storing the DNA compactly around the centre of the egg cell (left). Without Hira, the DNA disperses (right). (Credit: MRC Clinical Sciences Centre)
Each strand of DNA inside our body’s cells is nearly 2 metres long, and is wound around spool-like proteins, called histones, so that it can fit neatly inside a cell. It is these histones, stacked on top of each other in a pile eight high, that once packed alongside other architectural elements form the cell’s complex DNA storage structure – known as chromatin.
Once constructed, chromatin is not static. Ordinarily, when a cell needs to copy or repair its DNA, it temporarily opens up the chromatin by pulling the stacks of histones apart and unwinding these spool-like proteins. Once the repair or copying is complete, chaperone proteins move the histones back into place.
This process of opening and closing the chromatin happens constantly in most of the body’s cells, because they are active and growing, so need to copy and use their DNA a lot. All this activity makes it difficult for scientists to study how the chromatin is formed, and the exact role played by each of the chaperone proteins.
In this study, the researchers used mouse egg cells, called oocytes. Egg cells can grow in size but do not make copies of themselves like ‘normal’ body cells, allowing the researchers a clearer view of the chromatin’s activity.
The results, published this month in Molecular Cell, show that the chromatin in oocytes is in fact active and constantly changing – just as it is in the rest of the body’s cells – and that if this process goes wrong it dramatically affects oocyte viability and overall fertility.
“Oocytes can sit in the ovary for decades. We show that even in these resting, waiting oocytes the structure in which the DNA is stored is dynamic,” says Petra Hajkova, who leads the Reprogramming and Chromatin group at the Medical Research Council’s (MRC) Clinical Sciences Centre (CSC), based at Imperial College London.
Scientists at the CSC collaborated with others at the Babraham Institute in Cambridge and the University of Zurich in Switzerland. The team used mice genetically engineered so that they lacked the Hira “Histone regulatory A” chaperone.
The latest findings show that without Hira many of the oocytes developed abnormally or died. “Oocytes lacking the Hira histone chaperone showed severe developmental defects which often led to cell death. The whole system is disrupted, eggs accumulate DNA damage and the altered chromatin means that genes cannot be efficiently silenced or activated,” says Dr Gavin Kelsey, a research group leader in the Babraham Institute’s Epigenetics programme who collaborated with Hajkova’s team on the study.
“The beauty of this study is that it shows in animals that Hira is vital for how DNA is organised and packaged within the resting cell controlling how genes are switched ‘on’ and ‘off’ – disruption of this packaging machinery is catastrophic,” says Professor Richard Festenstein who also collaborated on the study, and who is head of the Gene Control Mechanisms and Disease Group within the Department of Medicine at Imperial College and Associate Researcher of the CSC.
The findings might help to explain why a woman’s fertility decreases with age. By the time a woman is thinking of having children her oocytes may have been sitting in her ovary for decades, Hajkova says. “If components of the structure in which DNA is stored are constantly changing, this turnover opens up the window for damage, so our work could explain why the quality of oocytes declines over time – in mice and in women.”
Read more on this from the Babraham Institute and Genetic Engineering and Biotechnology News.
For further information, contact:
Deborah Oakley
Science Communications Officer
MRC Clinical Sciences Centre
Du Cane Road
London W12 0NN
T: 0208 383 3791
M: 07711 016942
E:
