 Nick Myant: 50 years working for MRC at Hammersmith
Nick Myant: 50 years working for MRC at Hammersmith
A review in Proc. Royal Soc. B by Nick Myant – an associate of the CSC’s Lipoprotein Group who first worked for the MRC at Hammersmith in the 1950s – highlights a highly conserved amino acid sequence in the protein ApoER2, present in placental mammals but not marsupials, birds, or reptiles.
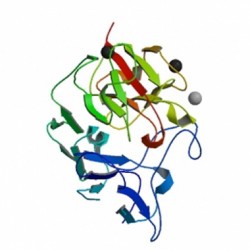
ApoER2 itself binds the protein reelin (structure shown at right), which plays an important role in a host of physiological processes. In the brain, it directs cortical layering. In mammals, layering proceeds in an ‘inside-out’ fashion: newly-created neurons pass from the germinal layer to the cortical plate through a sucession of increasingly younger neuronal layers, the oldest neurons being the most deeply-residing. Reptilian cortices, in contrast, feature their oldest constituent neurons on the outside (an architecture referred to as ‘outside-in’). Lack of reelin during development of the mammalian brain, such as was first observed in the spontaneously arising ‘reeler’ mutation in mouse populations (from whence the protein name is derived), leads to a layering pattern roughly reversed from that of healthy mammals (i.e. similar to ‘outside-in’). Mutations in the reelin gene have since been implicated as factors in schizophrenia, Alzheimer’s Disease and autism.
It has been suggested that the mammalian architecture may bestow an evolutionary advantage, in more readily facilitating brain volume increase. The Review considers whether or not this is due to the highly conserved sequence of amino acids in placental mammalian ApoER2 (and interaction with reelin), and the exon that encodes for it (exon 18 in primates, exon 19 in non-primates). Sequence comparisons of analogous regions, in both evolutionarily more distant amniotes and marsupials, reveal an absence of a homologue of exon 18 or 19, indicating that the new exon arose after the marsupial/placental mammalian split. But while mice lacking the exon exhibit diminished memory, spatial and motor function similar to that seen in the reeler mutation, cortical growth patterns remain a ‘healthy’ inside-out, ruling out the addition as being crucial to that particular brain architecture.
Link to the Review in Proceedings of the Royal Society B

