By Helen Figueira
July 8, 2013
Time to read: 7 minutes
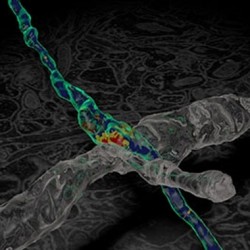 Watching neurons repair themselves in a living brain
Watching neurons repair themselves in a living brain
A slip of the kitchen knife can leave you one finger short of a full set for the rest of your life, but cut your hair and it (hopefully) grows back. Similarly, the neurons that sprawl throughout our body forming our peripheral nervous system – like the ones in your arm that allow your hands to move – are known to gradually regrow following damage. But those in our brain and spinal cord have never seemed so obliging. Or so we thought. New research from the CSC reveals that it’s not quite so clear-cut. A string of papers from the Neuroplasticity and Disease Group alleges that central nervous system neurons not only regrow, but might lead the way to harnessing our body’s natural self-healing potential.
If peripheral nerves are severed they can slowly inch down pre-laid tunnels and rebuild lost connections. Past research has shown, however, that similar injury to CNS nerves meets with factors that inhibit neuronal growth. And scar tissue in the CNS can present a barrier to regrowth. “So if we could prevent the formation of this scar, would axons regenerate in the brain?” asked Vincenzo de Paola. Using ultra-precise laser microsurgery to sever individual neurons in a mouse brain, his team minimised collateral damage sufficiently to prevent the scar tissue forming. By watching how the nerves subsequently behaved, they began to disentangle the relative roles of the inhibitory environment versus the intrinsic properties of the neurons in preventing regrowth.
“Using multiphoton in vivo imaging to track the regeneration of over a hundred neurons in an intact brain, we saw that some neurons regrow better than others,” explains De Paola. “There is an in-built difference between the various populations of neurons. What’s more, their regrowth can happen as fast as with peripheral nerves, but never follows the original trajectory of the nerve. Without the guidance molecules that were present months or years earlier when the brain first developed, the axons are lost. They do, however, make new connections with their surroundings. The question now is whether these fresh connections might be useful in restoring function.”
In addition to these fundamental discoveries, published in Nature Communications, studies published in the Journal of Neuroscience and PNAS use similar techniques to delve deeper into the phenomenon of axon regeneration and synaptic remodelling. It seems that neuron regrowth may be limited by the degree of retraction after the initial break. The greater this ‘dieback’, the less the potential for regrowth. Equally, particular neuron types seem prone to losing their connections, while the rate of their formation is unaltered. “To understand the dynamics of these processes there is no better way than monitoring them in real-time,” says De Paola.
He explains that both signalling pathways involved in the regulation of metabolism and epigenetic factors stimulate neuronal growth. Could they be used as a tool to kick-start the brain’s latent self-healing capacity? “Novel exciting techniques are now available to control the activity of injured neurons with unprecedented precision”, asserts De Paola. “We want to see if we can trigger regrowth in a more dramatic fashion than what we’ve shown already to understand more about the principles controlling regrowth and synaptic rewiring.” Armed with these new techniques and tantalising early revelations, the potential is growing.
Watching neurons repair themselves in a living brain
A slip of the kitchen knife can leave you one finger short of a full set for the rest of your life, but cut your hair and it (hopefully) grows back. Similarly, the neurons that sprawl throughout our body forming our peripheral nervous system – like the ones in your arm that allow your hands to move – are known to gradually regrow following damage. But those in our brain and spinal cord have never seemed so obliging. Or so we thought. New research from the CSC reveals that it’s not quite so clear-cut. A string of papers from the Neuroplasticity and Disease Group alleges that central nervous system neurons not only regrow, but might lead the way to harnessing our body’s natural self-healing potential.
If peripheral nerves are severed they can slowly inch down pre-laid tunnels and rebuild lost connections. Past research has shown, however, that similar injury to CNS nerves meets with factors that inhibit neuronal growth. And scar tissue in the CNS can present a barrier to regrowth. “So if we could prevent the formation of this scar, would axons regenerate in the brain?” asked Vincenzo de Paola. Using ultra-precise laser microsurgery to sever individual neurons in a mouse brain, his team minimised collateral damage sufficiently to prevent the scar tissue forming. By watching how the nerves subsequently behaved, they began to disentangle the relative roles of the inhibitory environment versus the intrinsic properties of the neurons in preventing regrowth.
“Using multiphoton in vivo imaging to track the regeneration of over a hundred neurons in an intact brain, we saw that some neurons regrow better than others,” explains De Paola. “There is an in-built difference between the various populations of neurons. What’s more, their regrowth can happen as fast as with peripheral nerves, but never follows the original trajectory of the nerve. Without the guidance molecules that were present months or years earlier when the brain first developed, the axons are lost. They do, however, make new connections with their surroundings. The question now is whether these fresh connections might be useful in restoring function.”
In addition to these fundamental discoveries, published in Nature Communications, studies published in the Journal of Neuroscience and PNAS use similar techniques to delve deeper into the phenomenon of axon regeneration and synaptic remodelling. It seems that neuron regrowth may be limited by the degree of retraction after the initial break. The greater this ‘dieback’, the less the potential for regrowth. Equally, particular neuron types seem prone to losing their connections, while the rate of their formation is unaltered. “To understand the dynamics of these processes there is no better way than monitoring them in real-time,” says De Paola.
He explains that both signalling pathways involved in the regulation of metabolism and epigenetic factors stimulate neuronal growth. Could they be used as a tool to kick-start the brain’s latent self-healing capacity? “Novel exciting techniques are now available to control the activity of injured neurons with unprecedented precision”, asserts De Paola. “We want to see if we can trigger regrowth in a more dramatic fashion than what we’ve shown already to understand more about the principles controlling regrowth and synaptic rewiring.” Armed with these new techniques and tantalising early revelations, the potential is growing.
References:
Canty, A.J., Huang, L., Jackson, J., Maco, B., Knott, G., Little, G., De Paola, V. (2013). In-vivo single neuron axotomy triggers axon regeneration to restore synaptic density in specific cortical circuits. Nature Communications Abstract (see also highlight in Nature)
Canty, A.J., Teles-Grilo Ruivo, L., Nesarajah, C., Jackson, J.S., Little, G., Song, S., De Paola, V (2013). Synaptic elimination and protection after minimal injury depend on cell type and their pre-lesion structural dynamics in the adult cerebral cortex. Journal of Neuroscience Abstract
Allegra Mascaro, A. L., Cesare, P., Sacconi, L., Grasselli, G., Mandolesi, G., Maco, B., Knott, G., Huang, L., De Paola, V., Strata, P., Pavone, F.S. (2013). In vivo single branch axotomy induces GAP-43 dependent sprouting and synaptic remodeling in cerebellar cortex. Proceedings of the National Academy of Sciences of the United States of America Abstract