 Relative gene expression levels determine cell fate
Relative gene expression levels determine cell fate
The emergence of bodily form out of an amorphous clump of cells in embryonic development is a field of enquiry with an auspicious provenance: from Aristotle’s arguments for epigenesis over preformatism, to Conrad Waddington’s groundwork for a modern theory of epigenetics in the 1940s; it has been tackled by some of the best minds in history, from a broad range of disciplines. In a 1952 paper, ‘The Chemical Basis of Morphogenesis’, the mathematician Alan Turing proposed that the form arise from a complex molecular dance of catalysis, inhibition and diffusion; the tiniest perturbations of a cell’s chemistry causing a cascade of events that give an embryo its shape. He called his theoretical agents ‘morphogens’, a term used today to describe biochemicals that influence transcription of DNA during embryonic development.
Two such morphogens, Nodal and Activin, are secreted from cells into the extracellular space – between adjacent cells – to control certain aspects of development and growth in many vertebrate species. Part of the TGF-β superfamily of signaling molecules – important in cell differentiation, proliferation and death – they influence the identity and fate of adjacent cells by passing information into the nucleus via the effectors Smad2 and Smad3. The effector Smads then transcribe a selection of target genes and assign a particular fate to a cell depending upon the morphogen concentration it is exposed to. The morphogen Nodal, for example, is crucial in mediating positional information in the developing embryo: High levels of nodal form the head, while low levels become the ‘trunk’, or torso.
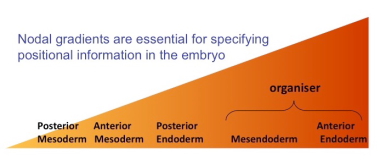
The mechanism by which morphogens convert quantitative information – a concentration – into a qualitatively singular fate – a cell type – has, however, remained elusive. It also wasn’t known whether different genes are switched on when morphogen levels are high compared to when they were low.
Work recently reported by the Mammalian Neurogenesis group at the CSC, led by Vasso Episkopou, aimed to address these questions by manipulating levels of Smad2/3 in embryonic stem cells. The group used a protocol including both an activator of Smad2/3, and a complementary inhibitor, which allowed them to control the level of activated Smad2/3 over time, and then analyse gene expression in microarrays to look for any effects.
The study identified several tens of genes, both known and novel, whose expression levels changed in step when the activation of Smad2/3 was manipulated. Morphogen levels directly influence the levels of transcription of the identified ‘target’ genes, suggesting that cell fate is determined not by a specific set of genes being switched on or off, but by relative expression levels of several target genes.
Because genes are transcribed at different rates – some quickly and some much more slowly – the researchers’ findings also indicate that length of exposure to a particular morphogen is as important as its concentration.
Only a few genes were found to be expressed solely due to Smads, with many target genes exhibiting a moderate level of expression even in the absence of Smad activation. Smads therefore work to reinforce expression, implying that cell fate is determined by threshold expression levels rather than a gene being just ‘on’ or ‘off’.
Insight into this mechanism is important, as disruption of the TGF-β pathway can lead to developmental diseases and cancer. The study reported also provides a new investigative tool for carrying out further research.
Stefan Janusz
Reference:
Guzman-Ayala M, Lee KL, Mavrakis KJ, Goggolidou P, Norris DP, Episkopou, V. (2009) Graded Smad2/3 Activation Is Converted Directly into Levels of Target Gene Expression in Embryonic Stem Cells. PLoS ONE 4(1): e4268. Link to PloS paper
