By Jianyi Nie
New insights by MRC-LMS researchers have shed light on the way that some anti-cancer drugs continue working even once a patient stops treatment. Although doctors had observed that three drugs given to treat metastatic breast cancer, Palbociclib, Abemaciclib and Ribociclib, had long-lasting effects, scientists did not understand why until now. Alexis Barr and her team discovered that these drugs, which all target enzymes known as CDK4 and CDK6 can put cancer cells into a permanent sleep, reducing the risk of the cancer recurring. This discovery offers the opportunity to improve patient outcomes through better therapy combinations.
“Normal cells grow and proliferate in a well-regulated way. Unfortunately, these processes go wrong in cancer” said Dr Alexis Barr, one of the lead authors of the study and leader of the MRC LMS Cell Cycle Control Team. “Our team has long been interested in understanding how cells control cycles of growth and proliferation. We want to understand this so that we can design better treatments for patients, or use existing treatments in better ways. We are excited about the insights gained from this study.”
CDK4 and CDK6 are the first enzymes to be activated when cells prepare to grow and proliferate. CDK4/6 inhibitor drugs, Palbociclib, Abemaciclib and Ribociclib, therefore, were designed to stop unregulated cell proliferation. Surprisingly, patients treated with the drugs discovered that when they stopped taking the CDK4/6 inhibitors, the cancer cells stayed ‘asleep’ and did not start proliferating again. Until now, however, the mechanisms underlying this were unknown. Researchers showed that while CDK4/6 inhibitors prevent cells from entering a new cycle of proliferation, these drugs do not stop cells from growing larger. As a result, cells become much bigger than they should. This cell “overgrowth” triggers stress responses in the cells which react by going to ‘sleep’ permanently.
 |
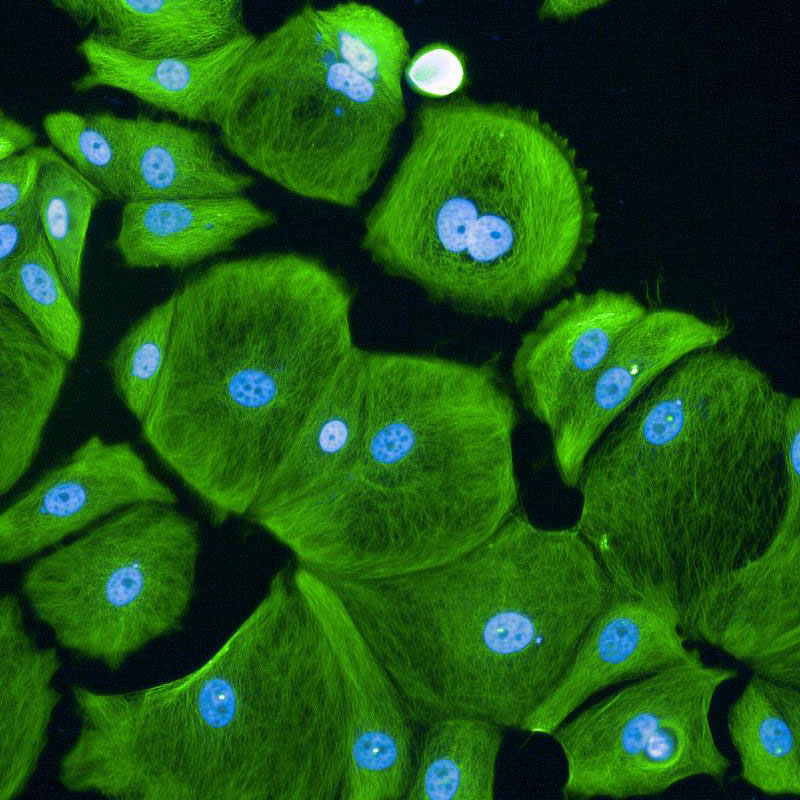 |
Images (Credit to Will Weston, a PhD student in the lab) show breast cancer cells either untreated (first image) or treated for 7 days (the second image) with CDK4/6 inhibitors. Cell sizes increase massively after being treated for 7 days.
Breast cancer has diverse subtypes. Patients with different subtypes respond differently to drugs. Therapies that work for some patients may not yield the same results for others. Some subtypes are characterized by specific genetic mutations. For example, p53 gene mutations are found in a subset of patients and these patients do not respond well to CDK4/6 inhibitor treatment. Excitingly, this research has helped explain why: when the p53 gene is mutated and non-functional, cells are not able to be put into permanent sleep by the drugs, and therefore, once treatment stops, cancer cells can start to proliferate again. Understanding this helps to identify cancer patients that could benefit from CDK4/6 inhibitor treatment.
Furthermore, the study offers new options that can be used as markers for treatment monitoring. The first marker is a protein called p21, which is one key player in this stress response. p21 prevents CDK4/6 from activating, thus making sure the cells stay asleep. Therefore, the level of p21 protein could serve as a marker to guide clinicians in assessing the CDK4/6 inhibitor’s effectiveness and determining whether to continue therapy. The second marker is the nuclear size. The study finds that when cells grow too big, their internal parts, like the nucleus, also change in size. Consequently, nuclear size, easily measured from biopsies, could also potentially be used as a marker to monitor treatment response.
“This important study details a novel mechanism through which CDK4/6 inhibitors can lead to durable withdrawal from cell division that has implications for therapeutic responses,” says Professor Erik Knudsen, Senior Vice President and Associate Director for Basic Science at the Roswell Park Comprehensive Cancer Center. The study, led by teams at the Medical Research Council Laboratory of Medical Sciences (MRC LMS) and the University of Dundee, was published on November 16th in Molecular Cell. Three additional studies, published in the same journal, from the Neurohr lab at the ETH in Zurich, the deBruin lab at UCL in London and the Saurin lab at the University of Dundee, drew similar conclusions and demonstrated the excitement around this topic. Read the study here.
Read our collaborators’ papers:
https://www.cell.com/molecular-cell/fulltext/S1097-2765(23)00855-9
https://www.cell.com/molecular-cell/fulltext/S1097-2765(23)00857-2
https://www.cell.com/molecular-cell/fulltext/S1097-2765(23)00854-7
