We are delighted to introduce our most recent group head, Dr Harry Leitch, who has recently established the Germline and Pluripotency group as part of the Epigenetics section at LMS. Harry has been awarded a three year Biotechnology and Biological Sciences Research Council (BBSRC) grant titled: Interrogating the potential of mouse primordial germ cells in vivo.
Harry has recently published a study in Cell Reports that reveals new insight into a protein called Nanog, which is key to early embryonic development. The study reveals that Nanog is not essential in germ cells as previously thought, and is functionally replaceable by an alternative gene, Esrrb.
Nanog and Esrrb are transcription factors, proteins that bind DNA and help to switch genes ‘on’ or ‘off’’. They have both previously been shown to play important roles in the development of embryonic stem cells (ESCs) and primordial germ cells (PGCs), the precursors to the sex cells. Nanog is a powerful regulator of pluripotency, the ability of one cell to become any of the hundreds of cell types in the adult body. It was identified in 2003 and named after Tir na nOg – the Celtic ‘Land of the Ever Young’ as it was shown to help maintain ESCs in an undifferentiated pluripotent state. Esrrb has also been shown to regulate pluripotency.
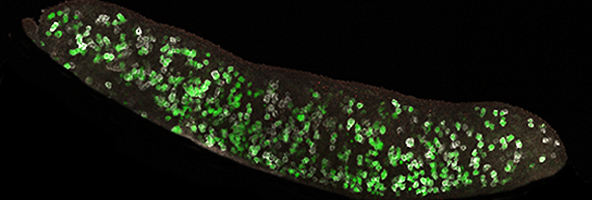
An E12.5 genital ridge showing Nanog-GFP and immunostained for the germ cell marker Dazl
Initial work suggested Nanog was an essential regulator of ESC pluripotency, however, in 2007 a landmark study demonstrated that Nanog could be deleted in ESCs, but, was apparently essential during PGC development.
Fast forward to today and this new study, a collaboration between Harry and the co-discoverer of Nanog Ian Chambers, from the MRC Centre for Regenerative Medicine in Edinburgh, shows that PGCs can actually develop in the absence of Nanog. While this study demonstrates that Nanog is non-essential, it does find that without it there is a severe reduction in the number of germ cells – with evidence of increased cell death and decreased cell division rate as possible causes of this.
Following the discovery that PGCs can continue to develop without Nanog, the authors went on to demonstrate that Esrrb can actually replace the function of Nanog in the germline. They modified the genome of mice so that Esrrb was switched on in place of Nanog – and these transgenic or genetically modified mice were found to have normal numbers of germ cells, providing clear evidence that the severe defects that arise when Nanog is removed can be rescued with the addition of Esrrb. This ability of Esrrb to compensate for Nanog in PGCs, in vivo, mirrors similar experiments in ESCs, in vitro.
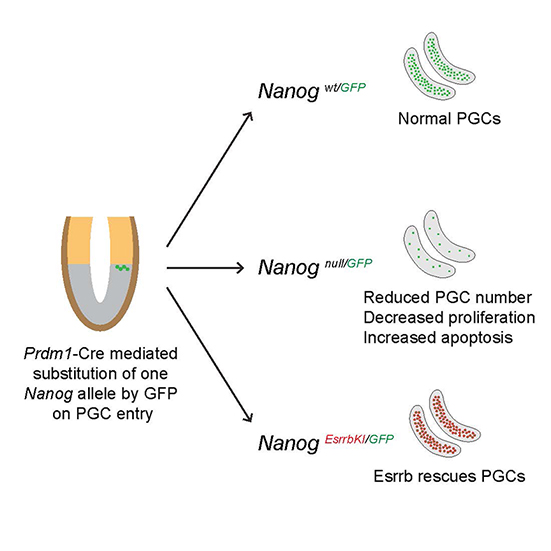
A graphical abstract depicting Esrrb complementation rescuing development of Nanog-null germ cells
So, are there any essential functions that remain for Nanog? Current evidence suggests that Nanog is still required for the development of pluripotent cells in the very early embryo and future work within this area could aid our understanding of Nanog’s role during this stage. Within this study the authors successfully re-wired the germ cells’ genetic network, opening up the idea of compensating for detrimental mutations by using completely different genes or pathways – rather than simply correcting faulty ones. This example suggests that a sufficient knowledge of any given cell type can allow the bypassing of defective pathways to rescue normal cell function.
In the context of newer technologies to edit the genome, such as CRISPR/Cas9, this has implications for how we go about engineering cells in vitro and possibly even in vivo. It implies that instructions within cells – even the crucial germ cells – are not written in stone, and can be modified should the need arise.
Read the full paper here.
