 Marshalling cell development to study Parkinson’s disease
Marshalling cell development to study Parkinson’s disease
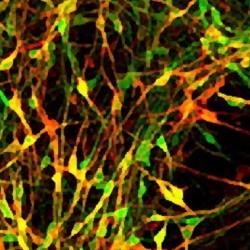
Every hour, someone in the UK is diagnosed with Parkinson’s disease. Slightly more than one in every hundred of us will succumb to this progressively debilitating neurological condition, for which there is currently no cure. Scientists don’t know why certain people get Parkinson’s disease, but it is known that a type of brain cell in the middle of the brain called dopamine neurons gradually die off.
Many scientists think that stem cells hold the key to successful treatment because they have the potential to become any cell in the body, including the midbrain neurons that die off in Parkinson’s disease. Producing dopamine nerve cells from stem cells will make it easier to monitor the effectiveness of potential new drugs that could slow or halt disease progression. With one of the most attractive advances of recent years – the introduction of induced pluripotent stem (iPS) cells which can be made from adult cells – it is now possible to make Parkinson’s neurons to study the disease in a dish.
Earlier this year the CSC Stem Cell Neurogenesis group published research showing that it is possible to generate midbrain dopamine neurons by ‘programming’ stem cells with a protein called Dmrt5, which is normally present inside cells that become dopamine neurons. Now, they’ve found a way to make them by stimulating the production of stem cells’ own Dmrt5. They did this by interfering with a chemical signalling system called FGF/ERK at defined stages of stem cells’ journey to becoming nerve cells, using a combination of small molecules and proteins. The FGF/ERK signalling system contributes to a cell’s ability to probe and correctly respond to its environment, telling it where to go and what to become; defining the location of the brain early on in embryonic development. By blocking and activating the signal at precisely the right time with cells grown in a dish, they can use this same signaling system to make midbrain dopamine neurons more simply, reliably and cheaply than by other methods.
Understanding the cues that direct the generation of desired cell types is fundamental for building up a ‘toolbox’ or repertoire of techniques to make cells for regenerative medicine. Importantly, the technique described by the CSC group works with iPS cells and EpiSCs – another recent advance that helps generate neurons more quickly – as well as embryonic stem cells, showing that the technique is widely applicable.
Dr Mark Ungless, head of CSC Neurophysiology and coauthor of the paper reflected on the success of the technique: “Importantly, these cells have many of the same electrophysiological characteristics that we see in functional dopaminergic neurons” – which shows that the technique really does work.
Ines Jaeger, the main author of the paper, explains: “In vitro-derived dopamine neurons provide a valuable tool for drug discovery and modelling of Parkinson’s disease. Because it is fully chemically-defined, this [method] could be readily adapted for use in a clinical setting, or scaled up for toxicity and drug screening relevant to developing new therapeutics for Parkinson’s disease.”
SJ Reference
Jaeger, I., Arber, C., Risner-Janiczek, J. R., Kuechler, J., Pritzsche, D., Chen, I.-C. C., Naveenan, T., Ungless, M. A., Li, M. (2011) Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells. Development, in press.
http://dx.doi.org/10.1242/dev.066746
