By Sofia Velazquez Pimentel
New research from the MRC LMS Epigenetic Memory Group has made significant advances in the use of bioluminescent reporter cells for understanding the epigenetic regulation of imprinted genes. These tools and approaches have the potential to aid the development of new epidrug therapies for diseases such as cancer and developmental disorders.
Epidrugs alter gene expression without changing the DNA sequence itself and are becoming an increasingly promising tool in disease treatment. For example, epidrugs may be able to block epigenetic modifying enzymes that promote cancer growth or could be used to activate genes to compensate for defects in developmental disorders.
The LMS Epigenetic Memory group, led by Professor Amanda Fisher, have previously developed tools to study imprinted genes. Each cell has two copies of each gene (one from each parent), but for imprinted genes only one copy is expressed whilst the other is silenced by epigenetic mechanisms. This unique quality makes imprinted genes, an unrivalled opportunity to test the effects of epidrugs on epigenetic silencing.
In their new publication in Scientific Reports, the team focused on the imprinted gene Cdk1nc, which is critical for growth and has a maternal copy that is expressed and a silenced paternal copy.
Building on previous work from the group, the researchers used a firefly luciferase gene in the DNA of mouse embryonic stem cells and fibroblast cells to report on the activity of Cdkn1c. These cells provide complementary tools to bioluminescent mice previously developed by the group, allowing screening and in-depth study of epidrugs. By partnering with GlaxoSmithKline, researchers were able to test the effect of different drugs on epigenetic silencing: if the epidrug successfully ‘awakened’ the paternal Cdkn1c gene then the cells would emit light. ‘This is an extremely sensitive method due to very little background signal. It is also convenient and non-invasive, allowing imaging of the same cells at different time points’ explained lead author Dr Andrew Dimond.
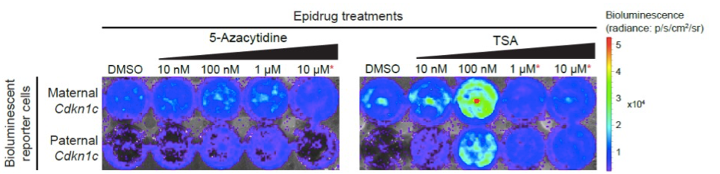
Bioluminescent reporter cells reveal epidrugs which effectively ‘awakened’ the paternal Cdkn1c gene.
Researchers identified several epidrugs which can disrupt epigenetic silencing of the normally silent paternal copy of the Cdkn1c gene. Strikingly, they found that the effects of these drugs were reversible, indicating that the epigenetic memory remained intact. As well as furthering our understanding of imprinted gene regulation, this new publication demonstrates that bioluminescent reporter cells can be used as tools for investigating epidrugs, including for drug screening.
‘Epidrugs represent a growing class of inhibitors, generated for eventual use in the clinic but challenges remain in selecting appropriate treatments, understanding their impacts and assessing the longevity of their effects. Our approach (using bioluminescent cells) can be used to screen and study epidrugs’ explained Dr Andrew Dimond, first author of the study, ‘Screening and studying epidrugs in different contexts may enable us to find or select better treatments for certain diseases by finding ways to switch on helpful genes or switch off problematic genes’.
The LMS Epigenetic Memory Group has made a significant contribution to the field of epidrug research with their new bioluminescent cell approach. Their method provides an important tool in the hunt for the best treatments for a range of diseases and could ultimately lead to more effective and safer therapies.

Dr Andrew Dimond, first author and researcher in the MRC LMS Epigenetic Memory Group using the team’s new bioluminescent tools
This research was published in Scientific Reporters and was carried out by the MRC LMS Epigenetic Memory group in collaboration with GlaxoSmithKline as well as MRC LMS WAPI, MRC LMS Microscopy Facility and the MRC LMS Lymphocyte Development group.
This research was funded by the Medical Research Council (MRC) and The Wellcome Trust.
